 Found 2151 hits Enz. Inhib. hit(s) with Target = 'Dihydrofolate reductase' AND taxid = 9606
Found 2151 hits Enz. Inhib. hit(s) with Target = 'Dihydrofolate reductase' AND taxid = 9606 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049905
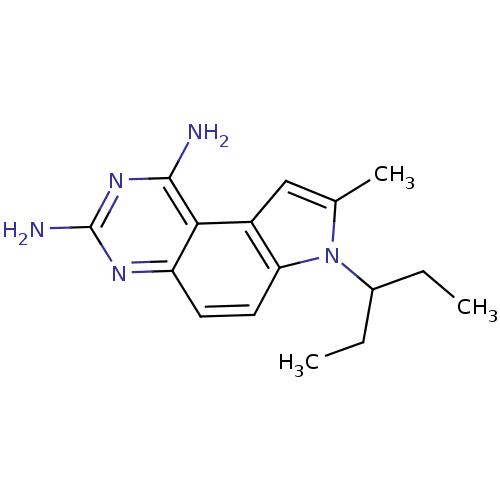
(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon Health& Science University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DHFR preincubated for 2 mins followed by substrate addition in presence of dihydrofolate |
Medchemcomm 6: 510-520 (2015)
BindingDB Entry DOI: 10.7270/Q2QN68NS |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50011320
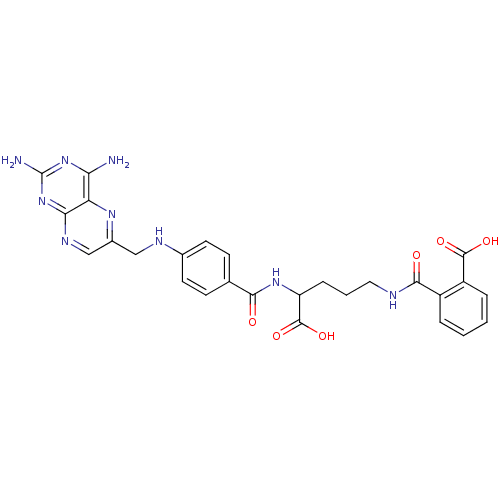
(CHEMBL18155 | N-(4-Carboxy-4-{4-[(2,4-diamino-pter...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C27H27N9O6/c28-21-20-22(36-27(29)35-21)32-13-16(33-20)12-31-15-9-7-14(8-10-15)23(37)34-19(26(41)42)6-3-11-30-24(38)17-4-1-2-5-18(17)25(39)40/h1-2,4-5,7-10,13,19,31H,3,6,11-12H2,(H,30,38)(H,34,37)(H,39,40)(H,41,42)(H4,28,29,32,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068813
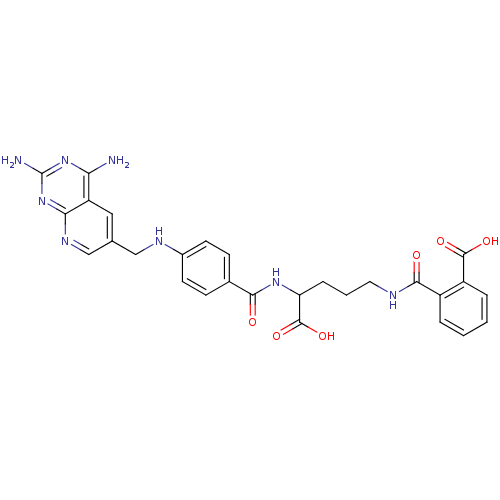
(CHEMBL149962 | N-(4-Carboxy-4-{4-[(2,4-diamino-pyr...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C28H28N8O6/c29-22-20-12-15(14-33-23(20)36-28(30)35-22)13-32-17-9-7-16(8-10-17)24(37)34-21(27(41)42)6-3-11-31-25(38)18-4-1-2-5-19(18)26(39)40/h1-2,4-5,7-10,12,14,21,32H,3,6,11,13H2,(H,31,38)(H,34,37)(H,39,40)(H,41,42)(H4,29,30,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068810
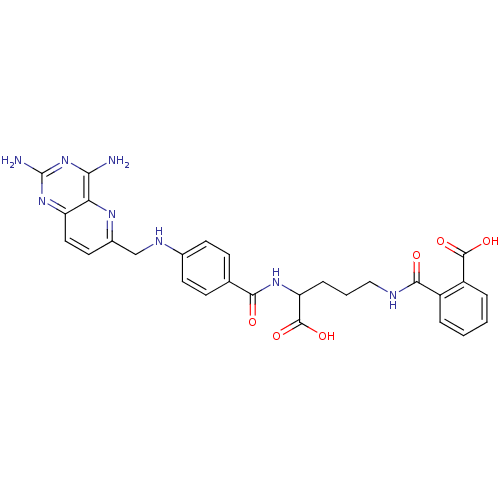
(CHEMBL149164 | N-(4-Carboxy-4-{4-[(2,4-diamino-pyr...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C28H28N8O6/c29-23-22-20(35-28(30)36-23)12-11-17(33-22)14-32-16-9-7-15(8-10-16)24(37)34-21(27(41)42)6-3-13-31-25(38)18-4-1-2-5-19(18)26(39)40/h1-2,4-5,7-12,21,32H,3,6,13-14H2,(H,31,38)(H,34,37)(H,39,40)(H,41,42)(H4,29,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068809
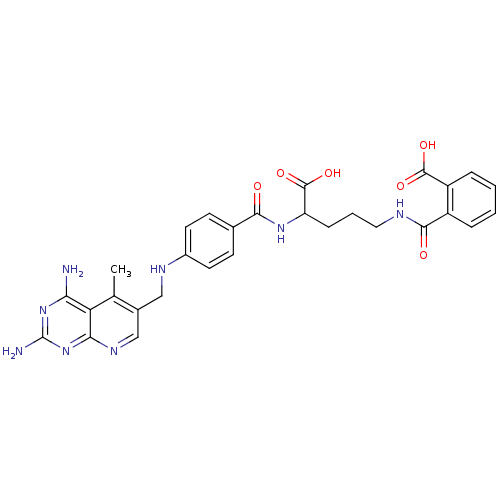
(CHEMBL150607 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-m...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCCNC(=O)c2ccccc2C(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C29H30N8O6/c1-15-17(14-34-24-22(15)23(30)36-29(31)37-24)13-33-18-10-8-16(9-11-18)25(38)35-21(28(42)43)7-4-12-32-26(39)19-5-2-3-6-20(19)27(40)41/h2-3,5-6,8-11,14,21,33H,4,7,12-13H2,1H3,(H,32,39)(H,35,38)(H,40,41)(H,42,43)(H4,30,31,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068811
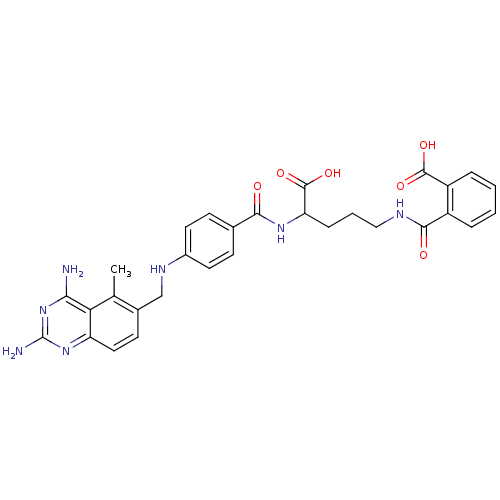
(CHEMBL149218 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-m...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCCNC(=O)c2ccccc2C(O)=O)C(O)=O)ccc2nc(N)nc(N)c12 Show InChI InChI=1S/C30H31N7O6/c1-16-18(10-13-22-24(16)25(31)37-30(32)36-22)15-34-19-11-8-17(9-12-19)26(38)35-23(29(42)43)7-4-14-33-27(39)20-5-2-3-6-21(20)28(40)41/h2-3,5-6,8-13,23,34H,4,7,14-15H2,1H3,(H,33,39)(H,35,38)(H,40,41)(H,42,43)(H4,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068808
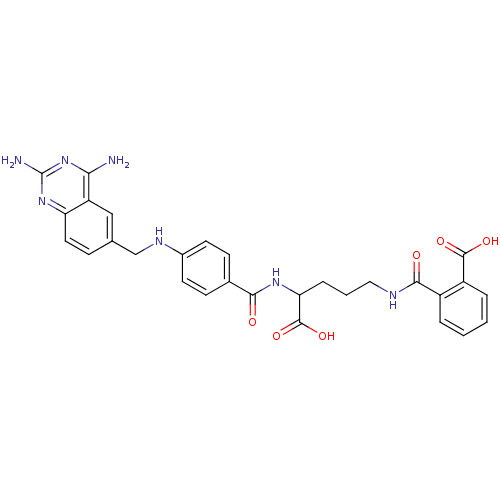
(CHEMBL297088 | N-(4-Carboxy-4-{4-[(2,4-diamino-qui...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H29N7O6/c30-24-21-14-16(7-12-22(21)35-29(31)36-24)15-33-18-10-8-17(9-11-18)25(37)34-23(28(41)42)6-3-13-32-26(38)19-4-1-2-5-20(19)27(39)40/h1-2,4-5,7-12,14,23,33H,3,6,13,15H2,(H,32,38)(H,34,37)(H,39,40)(H,41,42)(H4,30,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068812
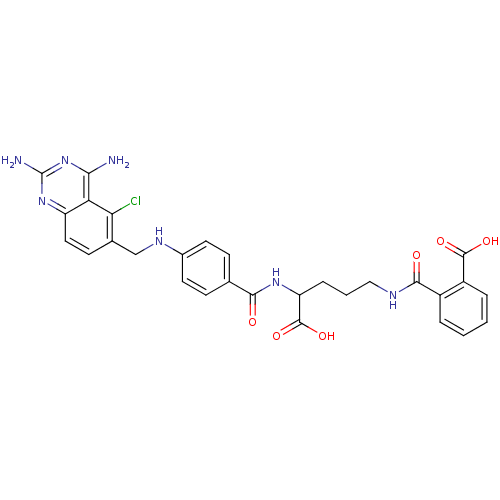
(CHEMBL146917 | N-(4-Carboxy-4-{4-[(2,4-diamino-5-c...)Show SMILES Nc1nc(N)c2c(Cl)c(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H28ClN7O6/c30-23-16(9-12-20-22(23)24(31)37-29(32)36-20)14-34-17-10-7-15(8-11-17)25(38)35-21(28(42)43)6-3-13-33-26(39)18-4-1-2-5-19(18)27(40)41/h1-2,4-5,7-12,21,34H,3,6,13-14H2,(H,33,39)(H,35,38)(H,40,41)(H,42,43)(H4,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50068808

(CHEMBL297088 | N-(4-Carboxy-4-{4-[(2,4-diamino-qui...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C29H29N7O6/c30-24-21-14-16(7-12-22(21)35-29(31)36-24)15-33-18-10-8-17(9-11-18)25(37)34-23(28(41)42)6-3-13-32-26(38)19-4-1-2-5-20(19)27(39)40/h1-2,4-5,7-12,14,23,33H,3,6,13,15H2,(H,32,38)(H,34,37)(H,39,40)(H,41,42)(H4,30,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0000140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111552
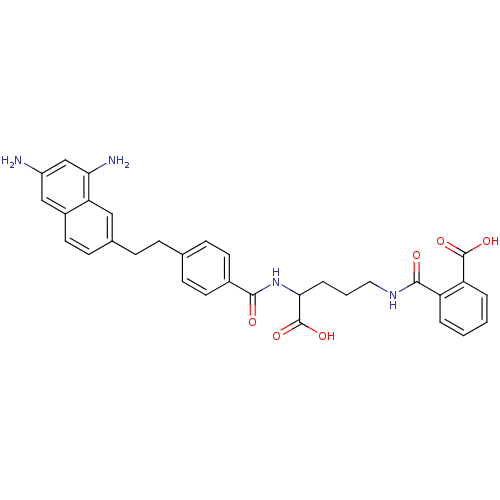
(CHEMBL297558 | N-(4-Carboxy-4-{4-[2-(6,8-diamino-n...)Show SMILES Nc1cc(N)c2cc(CCc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)ccc2c1 Show InChI InChI=1S/C32H32N4O6/c33-23-17-22-14-11-20(16-26(22)27(34)18-23)8-7-19-9-12-21(13-10-19)29(37)36-28(32(41)42)6-3-15-35-30(38)24-4-1-2-5-25(24)31(39)40/h1-2,4-5,9-14,16-18,28H,3,6-8,15,33-34H2,(H,35,38)(H,36,37)(H,39,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.000210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111550
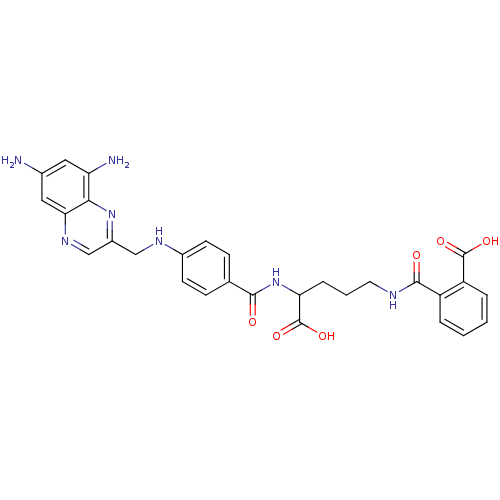
(CHEMBL296545 | N-(4-Carboxy-4-{4-[(6,8-diamino-qui...)Show SMILES Nc1cc(N)c2nc(CNc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2c1 Show InChI InChI=1S/C29H29N7O6/c30-17-12-22(31)25-24(13-17)34-15-19(35-25)14-33-18-9-7-16(8-10-18)26(37)36-23(29(41)42)6-3-11-32-27(38)20-4-1-2-5-21(20)28(39)40/h1-2,4-5,7-10,12-13,15,23,33H,3,6,11,14,30-31H2,(H,32,38)(H,36,37)(H,39,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.000330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.000340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR by spectrophotometric analysis |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111549
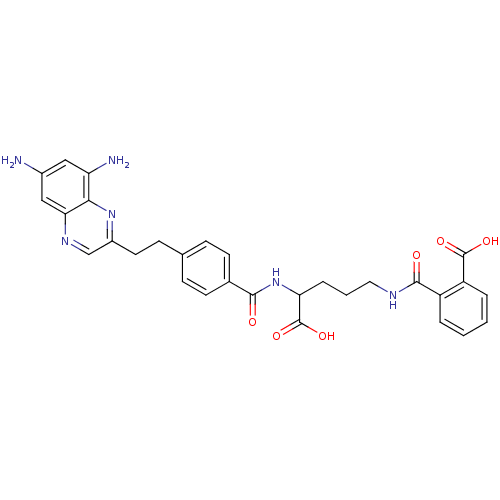
(CHEMBL47689 | N-(4-Carboxy-4-{4-[2-(6,8-diamino-qu...)Show SMILES Nc1cc(N)c2nc(CCc3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2c1 Show InChI InChI=1S/C30H30N6O6/c31-19-14-23(32)26-25(15-19)34-16-20(35-26)12-9-17-7-10-18(11-8-17)27(37)36-24(30(41)42)6-3-13-33-28(38)21-4-1-2-5-22(21)29(39)40/h1-2,4-5,7-8,10-11,14-16,24H,3,6,9,12-13,31-32H2,(H,33,38)(H,36,37)(H,39,40)(H,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111548

(CHEMBL47919 | N-(4-Carboxy-4-{4-[1-(6,8-diamino-qu...)Show SMILES Nc1cc(N)c2nc(CC(CC#C)c3ccc(cc3)C(=O)NC(CCCNC(=O)c3ccccc3C(O)=O)C(O)=O)cnc2c1 Show InChI InChI=1S/C33H32N6O6/c1-2-6-21(15-23-18-37-28-17-22(34)16-26(35)29(28)38-23)19-10-12-20(13-11-19)30(40)39-27(33(44)45)9-5-14-36-31(41)24-7-3-4-8-25(24)32(42)43/h1,3-4,7-8,10-13,16-18,21,27H,5-6,9,14-15,34-35H2,(H,36,41)(H,39,40)(H,42,43)(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50111551

(CHEMBL295859 | N-(4-Carboxy-4-{4-[1-(6,8-diamino-q...)Show SMILES CCC(Cc1cnc2cc(N)cc(N)c2n1)c1ccc(cc1)C(=O)NC(CCCNC(=O)c1ccccc1C(O)=O)C(O)=O Show InChI InChI=1S/C32H34N6O6/c1-2-18(14-22-17-36-27-16-21(33)15-25(34)28(27)37-22)19-9-11-20(12-10-19)29(39)38-26(32(43)44)8-5-13-35-30(40)23-6-3-4-7-24(23)31(41)42/h3-4,6-7,9-12,15-18,26H,2,5,8,13-14,33-34H2,1H3,(H,35,40)(H,38,39)(H,41,42)(H,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.000620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Dihydrofolate reductase |
J Med Chem 45: 1690-6 (2002)
BindingDB Entry DOI: 10.7270/Q2V987CB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate by fluorescence spectrophotometric ana... |
ACS Med Chem Lett 7: 692-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00120
BindingDB Entry DOI: 10.7270/Q2DJ5HKK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50288820
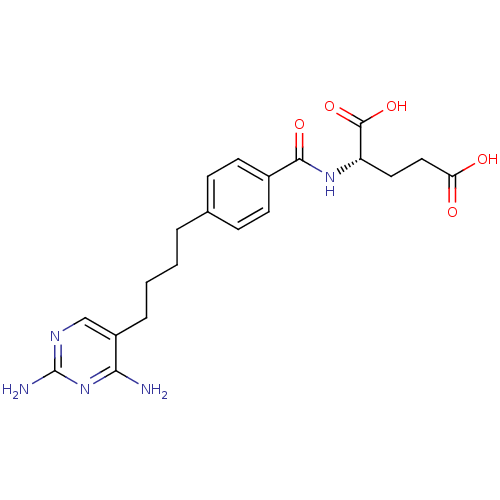
((S)-2-{(S)-4-[4-(2,4-Diamino-pyrimidin-5-yl)-butyl...)Show SMILES Nc1ncc(CCCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C20H25N5O5/c21-17-14(11-23-20(22)25-17)4-2-1-3-12-5-7-13(8-6-12)18(28)24-15(19(29)30)9-10-16(26)27/h5-8,11,15H,1-4,9-10H2,(H,24,28)(H,26,27)(H,29,30)(H4,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dihydrofolate reductase at concentration ranged from 0.15-0.50 uM |
J Med Chem 41: 5310-9 (1999)
Article DOI: 10.1021/jm980477+
BindingDB Entry DOI: 10.7270/Q2542P85 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research and Development, LLC
Curated by ChEMBL
| Assay Description
Binding affinity to DHFR (unknown origin) by NMR analysis |
J Med Chem 57: 7819-37 (2014)
Article DOI: 10.1021/jm500325k
BindingDB Entry DOI: 10.7270/Q2JW8GH3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50026273
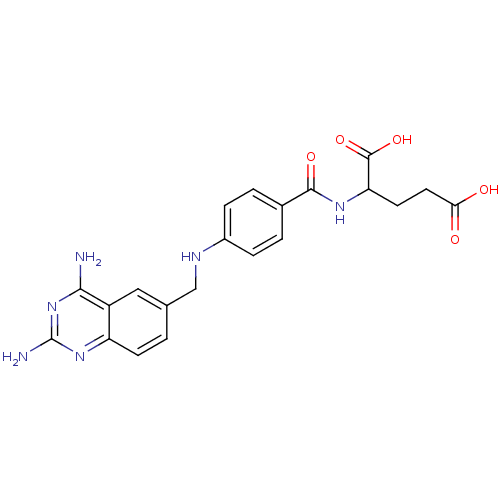
(2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-14-9-11(1-6-15(14)26-21(23)27-18)10-24-13-4-2-12(3-5-13)19(30)25-16(20(31)32)7-8-17(28)29/h1-6,9,16,24H,7-8,10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia, UniversitÓ di Genova, Viale Benedetto XV 3, 16132 Genova, Italy. Electronic address: tonelli@difar.unige.it.
Curated by ChEMBL
| Assay Description
Inhibition of DHFR (unknown origin) |
Eur J Med Chem 135: 467-478 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.070
BindingDB Entry DOI: 10.7270/Q2057JD0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50026273

(2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-14-9-11(1-6-15(14)26-21(23)27-18)10-24-13-4-2-12(3-5-13)19(30)25-16(20(31)32)7-8-17(28)29/h1-6,9,16,24H,7-8,10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50457437
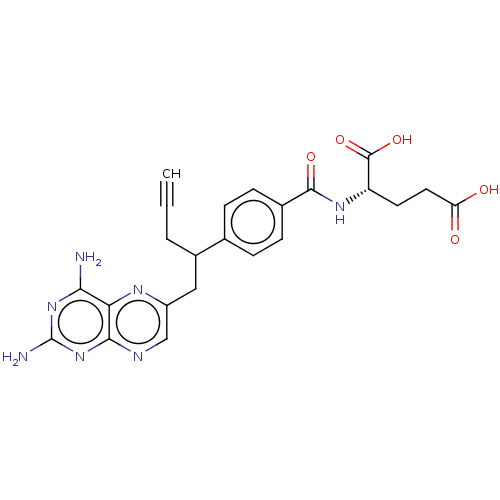
(CHEBI:71223 | Folotyn | PDX | Pralatrexate)Show SMILES [H][C@@](CCC(O)=O)(NC(=O)c1ccc(cc1)C(CC#C)Cc1cnc2nc(N)nc(N)c2n1)C(O)=O |r| Show InChI InChI=1S/C23H23N7O5/c1-2-3-14(10-15-11-26-20-18(27-15)19(24)29-23(25)30-20)12-4-6-13(7-5-12)21(33)28-16(22(34)35)8-9-17(31)32/h1,4-7,11,14,16H,3,8-10H2,(H,28,33)(H,31,32)(H,34,35)(H4,24,25,26,29,30)/t14?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113218
BindingDB Entry DOI: 10.7270/Q2PN99PG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50158569
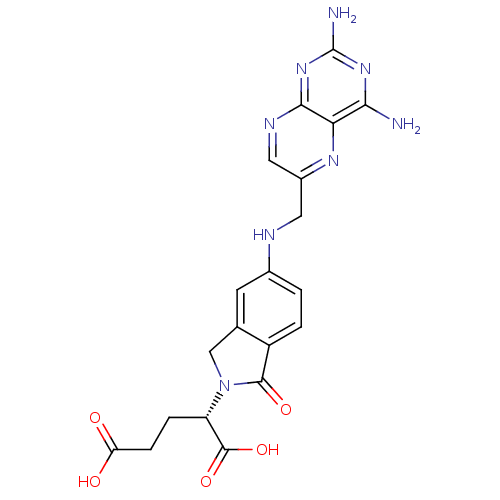
(2-S-[5-[2,4-diaminopteridin-6-yl)methyamino]-2,3-d...)Show SMILES Nc1nc(N)c2nc(CNc3ccc4C(=O)N(Cc4c3)[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C20H20N8O5/c21-16-15-17(27-20(22)26-16)24-7-11(25-15)6-23-10-1-2-12-9(5-10)8-28(18(12)31)13(19(32)33)3-4-14(29)30/h1-2,5,7,13,23H,3-4,6,8H2,(H,29,30)(H,32,33)(H4,21,22,24,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR |
J Med Chem 47: 6958-63 (2004)
Article DOI: 10.1021/jm040122s
BindingDB Entry DOI: 10.7270/Q2NZ873F |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase |
Bioorg Med Chem Lett 9: 1463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ657V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113016
BindingDB Entry DOI: 10.7270/Q28W3JD6 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50158570
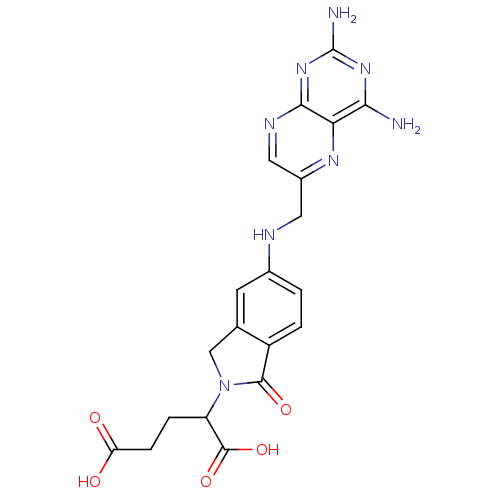
(2-R,S-[5-[(2,4-diaminopteridin-6-yl)methylamino]-2...)Show SMILES Nc1nc(N)c2nc(CNc3ccc4C(=O)N(Cc4c3)C(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H20N8O5/c21-16-15-17(27-20(22)26-16)24-7-11(25-15)6-23-10-1-2-12-9(5-10)8-28(18(12)31)13(19(32)33)3-4-14(29)30/h1-2,5,7,13,23H,3-4,6,8H2,(H,29,30)(H,32,33)(H4,21,22,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR |
J Med Chem 47: 6958-63 (2004)
Article DOI: 10.1021/jm040122s
BindingDB Entry DOI: 10.7270/Q2NZ873F |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049901
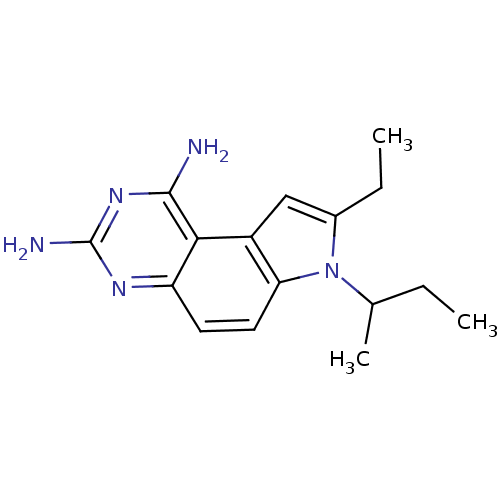
(7-sec-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-1...)Show InChI InChI=1S/C16H21N5/c1-4-9(3)21-10(5-2)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-9H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049906
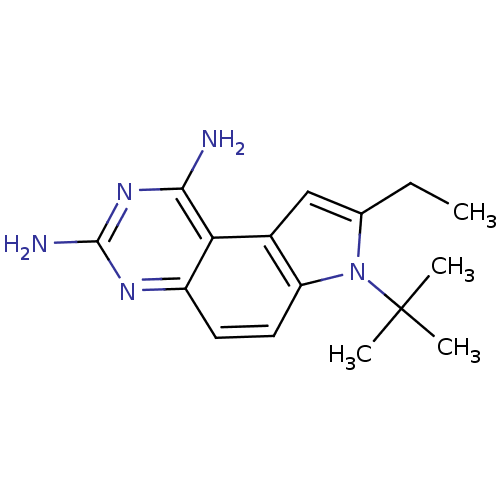
(7-tert-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-...)Show InChI InChI=1S/C16H21N5/c1-5-9-8-10-12(21(9)16(2,3)4)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049907

(7-(1-Ethyl-propyl)-8-isopropyl-7H-pyrrolo[3,2-f]qu...)Show InChI InChI=1S/C18H25N5/c1-5-11(6-2)23-14-8-7-13-16(17(19)22-18(20)21-13)12(14)9-15(23)10(3)4/h7-11H,5-6H2,1-4H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50077798
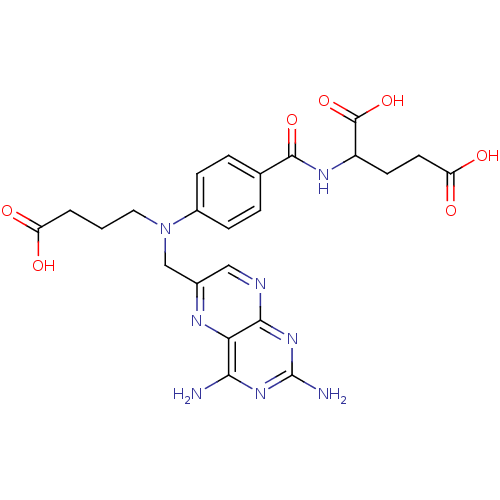
(2-{4-[(3-Carboxy-propyl)-(2,4-diamino-pteridin-6-y...)Show SMILES Nc1nc(N)c2nc(CN(CCCC(O)=O)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C23H26N8O7/c24-19-18-20(30-23(25)29-19)26-10-13(27-18)11-31(9-1-2-16(32)33)14-5-3-12(4-6-14)21(36)28-15(22(37)38)7-8-17(34)35/h3-6,10,15H,1-2,7-9,11H2,(H,28,36)(H,32,33)(H,34,35)(H,37,38)(H4,24,25,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase |
Bioorg Med Chem Lett 9: 1463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ657V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50288821

((S)-2-{(S)-4-[3-(2,4-Diamino-pyrimidin-5-yl)-propy...)Show SMILES Nc1ncc(CCCc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(N)n1 Show InChI InChI=1S/C19H23N5O5/c20-16-13(10-22-19(21)24-16)3-1-2-11-4-6-12(7-5-11)17(27)23-14(18(28)29)8-9-15(25)26/h4-7,10,14H,1-3,8-9H2,(H,23,27)(H,25,26)(H,28,29)(H4,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against Recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 6: 473-476 (1996)
Article DOI: 10.1016/0960-894X(96)00053-4
BindingDB Entry DOI: 10.7270/Q2GQ6XQ2 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50077796
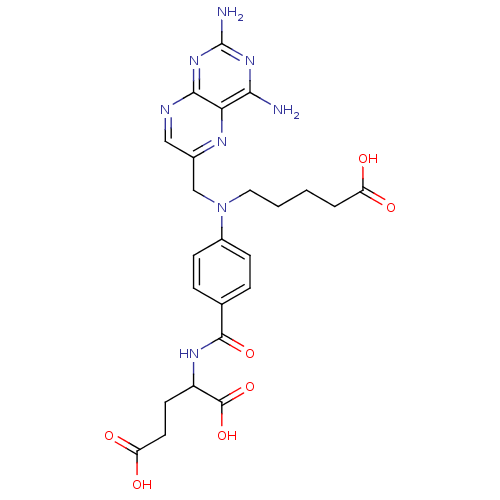
(2-{4-[(4-Carboxy-butyl)-(2,4-diamino-pteridin-6-yl...)Show SMILES Nc1nc(N)c2nc(CN(CCCCC(O)=O)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C24H28N8O7/c25-20-19-21(31-24(26)30-20)27-11-14(28-19)12-32(10-2-1-3-17(33)34)15-6-4-13(5-7-15)22(37)29-16(23(38)39)8-9-18(35)36/h4-7,11,16H,1-3,8-10,12H2,(H,29,37)(H,33,34)(H,35,36)(H,38,39)(H4,25,26,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase |
Bioorg Med Chem Lett 9: 1463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ657V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 0.179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase in humans |
Bioorg Med Chem Lett 9: 1463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ657V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50077799
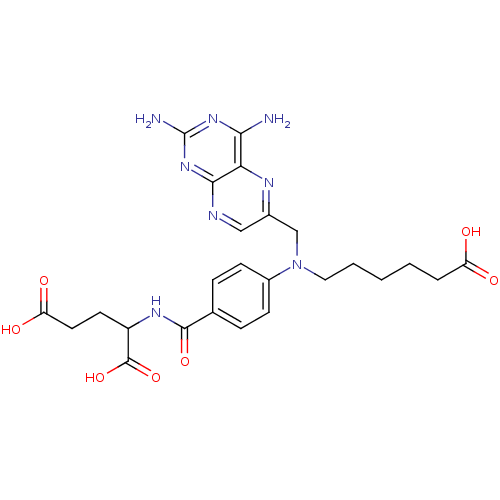
(2-{4-[(5-Carboxy-pentyl)-(2,4-diamino-pteridin-6-y...)Show SMILES Nc1nc(N)c2nc(CN(CCCCCC(O)=O)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cnc2n1 Show InChI InChI=1S/C25H30N8O7/c26-21-20-22(32-25(27)31-21)28-12-15(29-20)13-33(11-3-1-2-4-18(34)35)16-7-5-14(6-8-16)23(38)30-17(24(39)40)9-10-19(36)37/h5-8,12,17H,1-4,9-11,13H2,(H,30,38)(H,34,35)(H,36,37)(H,39,40)(H4,26,27,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase |
Bioorg Med Chem Lett 9: 1463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ657V |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049909

(8-tert-Butyl-7-isopropyl-7H-pyrrolo[3,2-f]quinazol...)Show InChI InChI=1S/C17H23N5/c1-9(2)22-12-7-6-11-14(15(18)21-16(19)20-11)10(12)8-13(22)17(3,4)5/h6-9H,1-5H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049897

(8-Ethyl-7-(1-ethyl-propyl)-7H-pyrrolo[3,2-f]quinaz...)Show InChI InChI=1S/C17H23N5/c1-4-10(5-2)22-11(6-3)9-12-14(22)8-7-13-15(12)16(18)21-17(19)20-13/h7-10H,4-6H2,1-3H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50073754
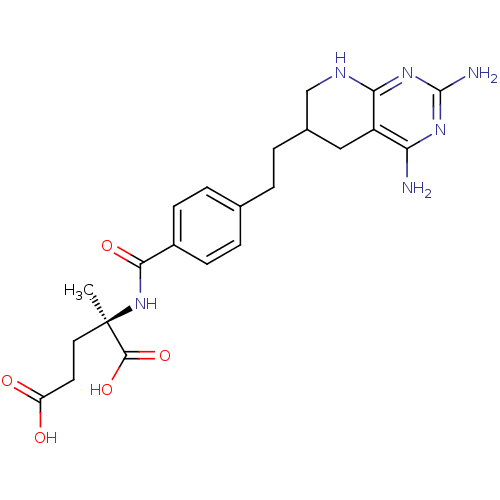
((R)-2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)cc1)C(O)=O Show InChI InChI=1S/C22H28N6O5/c1-22(20(32)33,9-8-16(29)30)28-19(31)14-6-4-12(5-7-14)2-3-13-10-15-17(23)26-21(24)27-18(15)25-11-13/h4-7,13H,2-3,8-11H2,1H3,(H,28,31)(H,29,30)(H,32,33)(H5,23,24,25,26,27)/t13?,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049905

(7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C16H21N5/c1-4-10(5-2)21-9(3)8-11-13(21)7-6-12-14(11)15(17)20-16(18)19-12/h6-8,10H,4-5H2,1-3H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049903
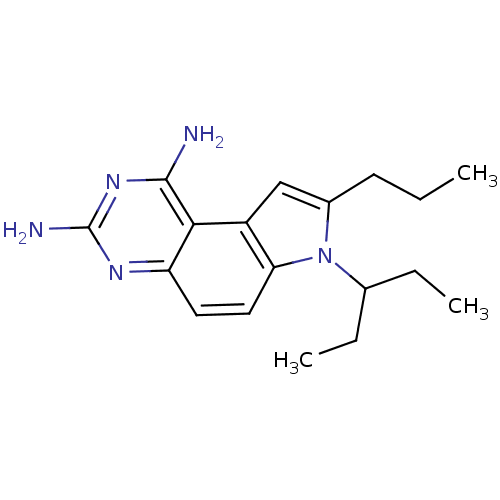
(7-(1-Ethyl-propyl)-8-propyl-7H-pyrrolo[3,2-f]quina...)Show InChI InChI=1S/C18H25N5/c1-4-7-12-10-13-15(23(12)11(5-2)6-3)9-8-14-16(13)17(19)22-18(20)21-14/h8-11H,4-7H2,1-3H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50049912

(7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...)Show InChI InChI=1S/C16H21N5/c1-5-16(3,4)21-9(2)8-10-12(21)7-6-11-13(10)14(17)20-15(18)19-11/h6-8H,5H2,1-4H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Dihydrofolate reductase enzyme |
J Med Chem 39: 892-903 (1996)
Article DOI: 10.1021/jm9505122
BindingDB Entry DOI: 10.7270/Q2XD10RC |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036495
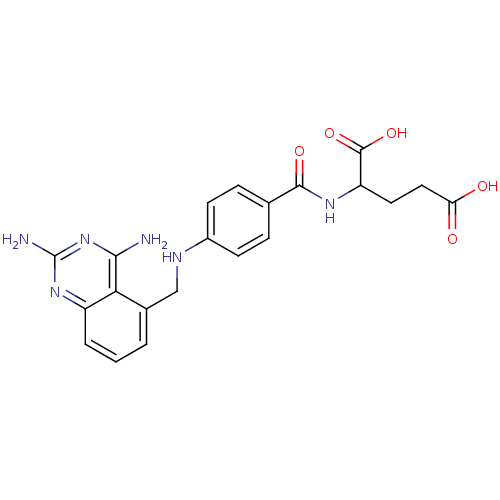
(2-{4-[(2,4-Diamino-quinazolin-5-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-17-12(2-1-3-14(17)26-21(23)27-18)10-24-13-6-4-11(5-7-13)19(30)25-15(20(31)32)8-9-16(28)29/h1-7,15,24H,8-10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50073752
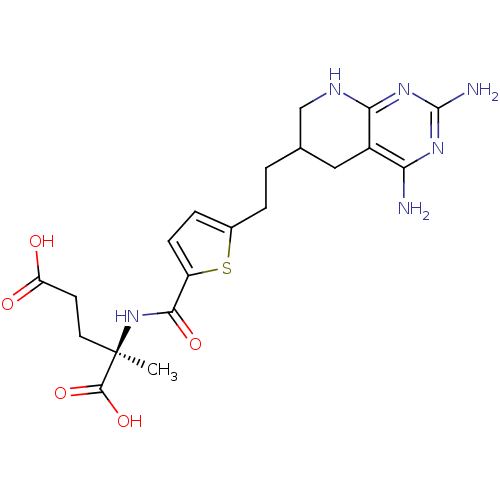
((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...)Show SMILES C[C@](CCC(O)=O)(NC(=O)c1ccc(CCC2CNc3nc(N)nc(N)c3C2)s1)C(O)=O Show InChI InChI=1S/C20H26N6O5S/c1-20(18(30)31,7-6-14(27)28)26-17(29)13-5-4-11(32-13)3-2-10-8-12-15(21)24-19(22)25-16(12)23-9-10/h4-5,10H,2-3,6-9H2,1H3,(H,26,29)(H,27,28)(H,30,31)(H5,21,22,23,24,25)/t10?,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) |
Bioorg Med Chem Lett 9: 75-8 (1999)
BindingDB Entry DOI: 10.7270/Q2SX6CCT |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50018469
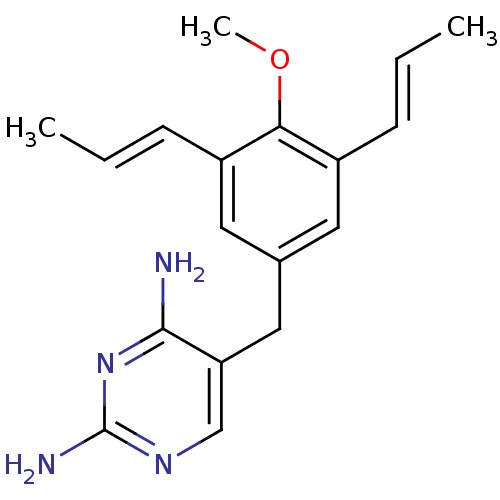
(5-(4-Methoxy-3,5-dipropenyl-benzyl)-pyrimidine-2,4...)Show InChI InChI=1S/C18H22N4O/c1-4-6-13-8-12(9-14(7-5-2)16(13)23-3)10-15-11-21-18(20)22-17(15)19/h4-9,11H,10H2,1-3H3,(H4,19,20,21,22)/b6-4+,7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli |
J Med Chem 32: 1949-58 (1989)
BindingDB Entry DOI: 10.7270/Q2DF6Q6G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data