

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (RAT) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to Endothelin A receptor in the rat atrium | J Med Chem 35: 1493-508 (1992) BindingDB Entry DOI: 10.7270/Q27M08KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50051007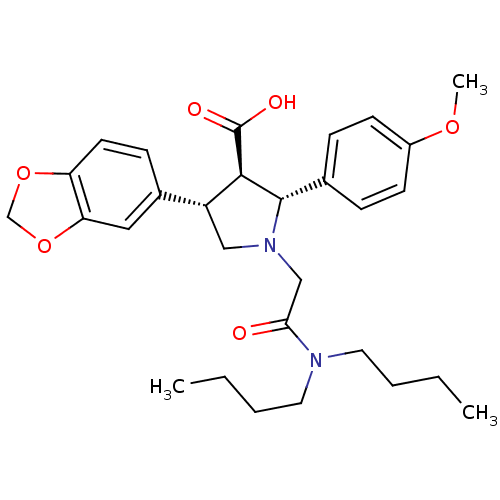 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards Endothelin A receptor | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50143784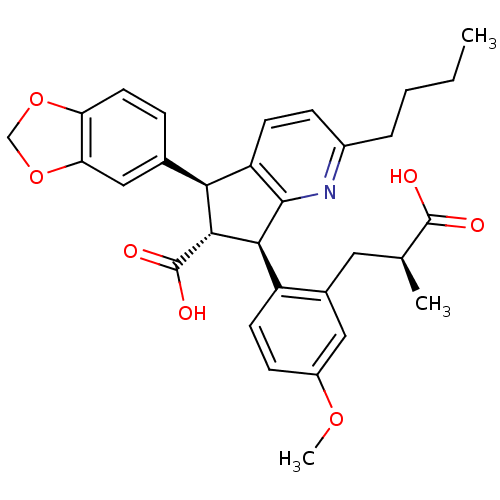 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards Endothelin A receptor | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50051007 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description binding affinity against human Endothelin A receptor | J Med Chem 44: 3978-84 (2001) BindingDB Entry DOI: 10.7270/Q2JS9PR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50368606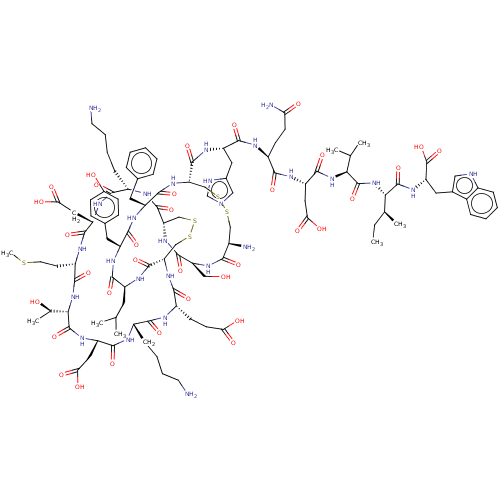 (Sarafotoxin S6B) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to Endothelin A receptor in the rat atrium | J Med Chem 35: 1493-508 (1992) BindingDB Entry DOI: 10.7270/Q27M08KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to Endothelin A receptor in the rat aorta | J Med Chem 35: 1493-508 (1992) BindingDB Entry DOI: 10.7270/Q27M08KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50368606 (Sarafotoxin S6B) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to Endothelin A receptor in the rat atrium | J Med Chem 35: 1493-508 (1992) BindingDB Entry DOI: 10.7270/Q27M08KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50058126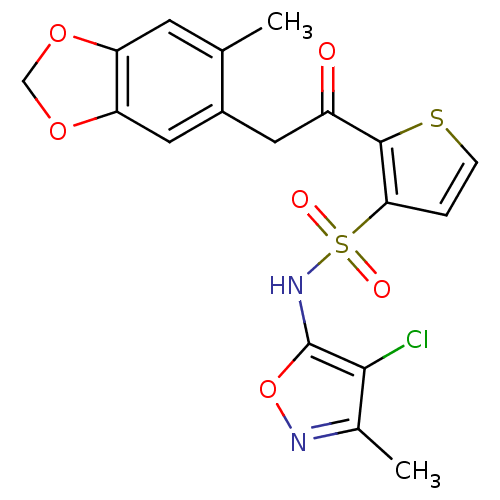 (2-[2-(6-Methyl-benzo[1,3]dioxol-5-yl)-acetyl]-thio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards Endothelin A receptor | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-ET1 from ETA receptor in A10 rat thoracic aorta smooth muscle after 3 hrs by scintillation counting method | Bioorg Med Chem Lett 26: 3381-94 (2016) Article DOI: 10.1016/j.bmcl.2016.06.014 BindingDB Entry DOI: 10.7270/Q26113SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-ET1 from ETA receptor in A10 rat thoracic aorta smooth muscle after 3 hrs by scintillation counting method | Bioorg Med Chem Lett 26: 3381-94 (2016) Article DOI: 10.1016/j.bmcl.2016.06.014 BindingDB Entry DOI: 10.7270/Q26113SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards Endothelin A receptor | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068673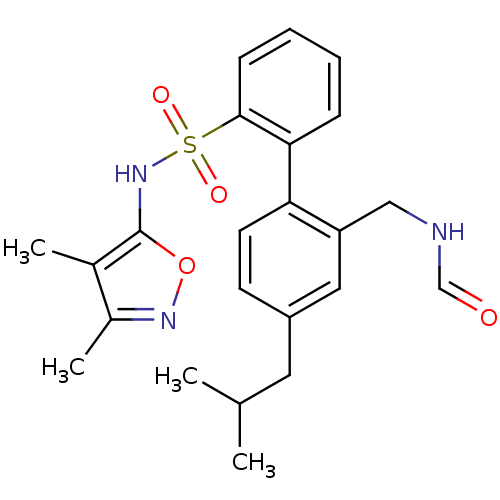 (2'-Formylaminomethyl-4'-isobutyl-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50368607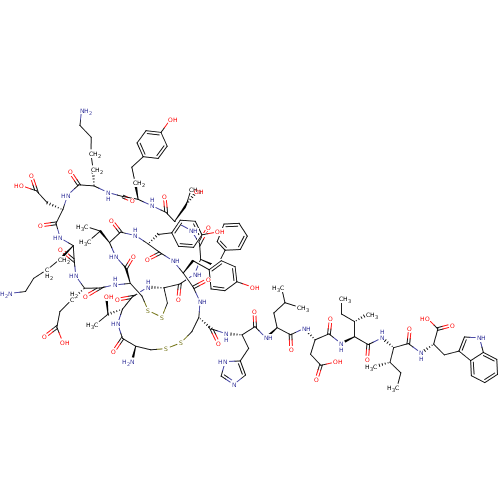 (CHEMBL1790180) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to Endothelin A receptor in the rat aorta | J Med Chem 35: 1493-508 (1992) BindingDB Entry DOI: 10.7270/Q27M08KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50368607 (CHEMBL1790180) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity to Endothelin A receptor in the rat aorta | J Med Chem 35: 1493-508 (1992) BindingDB Entry DOI: 10.7270/Q27M08KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068706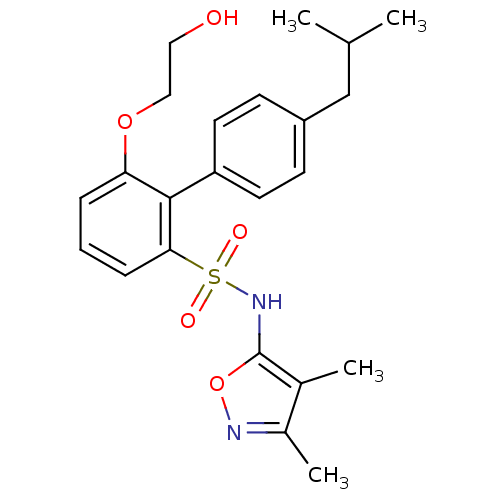 (6-(2-Hydroxy-ethoxy)-4'-isobutyl-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068674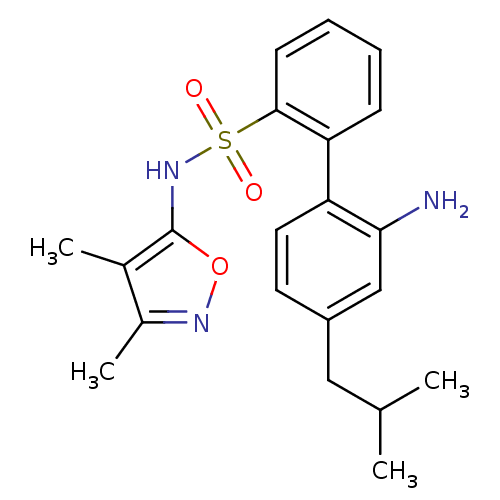 (2'-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068713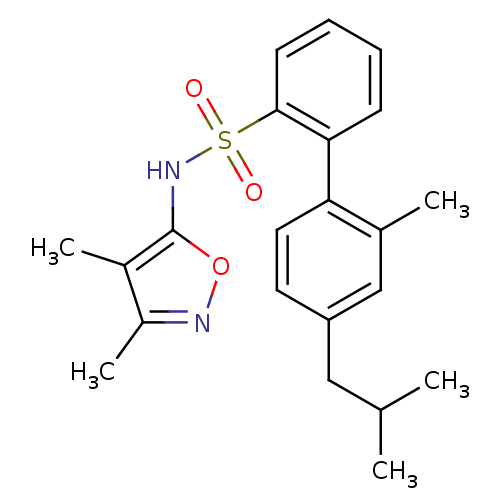 (4'-Isobutyl-2'-methyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068720 (6-Hydroxy-4'-isobutyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50108202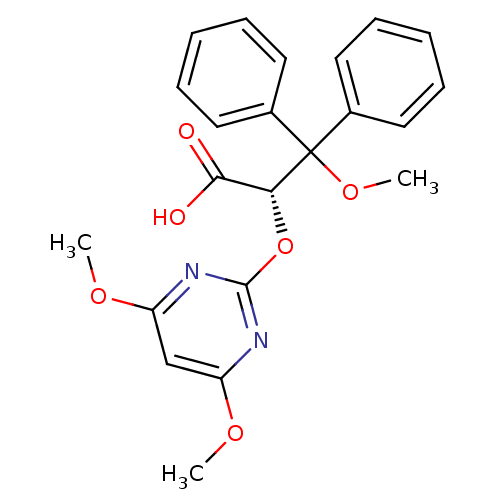 ((S)-2-(4,6-Dimethoxy-pyrimidin-2-yloxy)-3-methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards Endothelin A receptor | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068740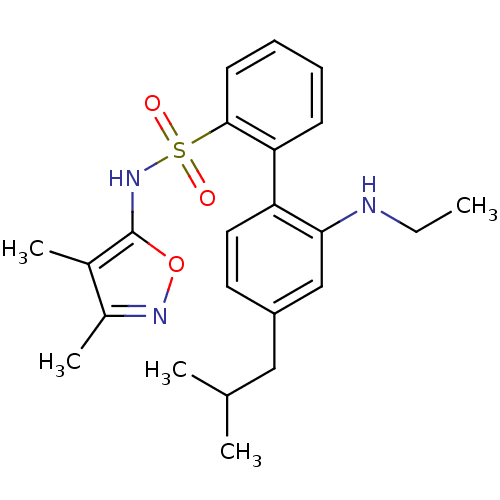 (2'-Ethylamino-4'-isobutyl-biphenyl-2-sulfonic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068697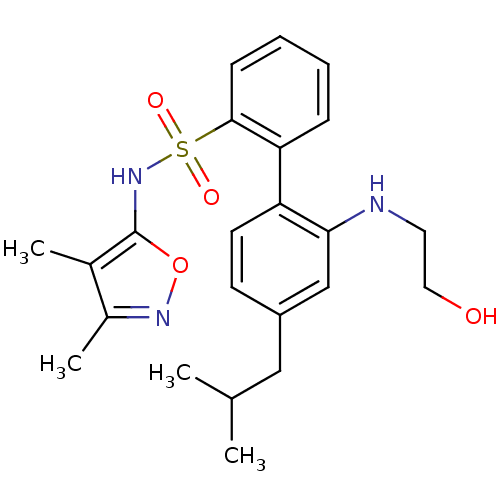 (2'-(2-Hydroxy-ethylamino)-4'-isobutyl-biphenyl-2-s...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50061101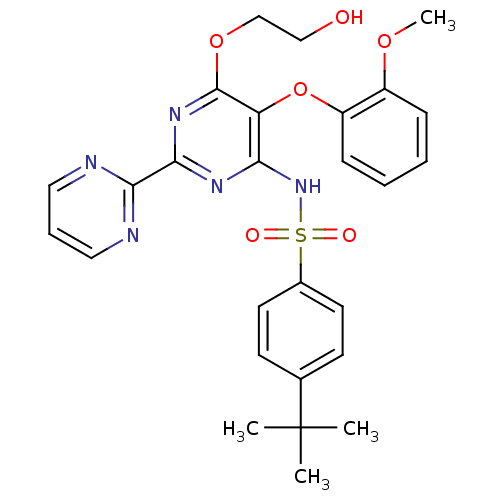 (4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity towards Endothelin A receptor | J Med Chem 47: 1969-86 (2004) Article DOI: 10.1021/jm030528p BindingDB Entry DOI: 10.7270/Q2HD7WD6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068736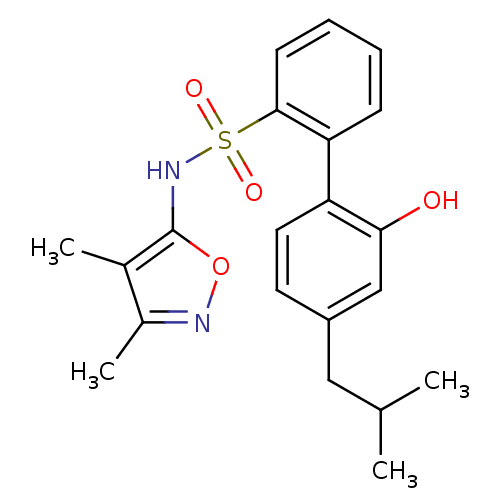 (2'-Hydroxy-4'-isobutyl-biphenyl-2-sulfonic acid (3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068685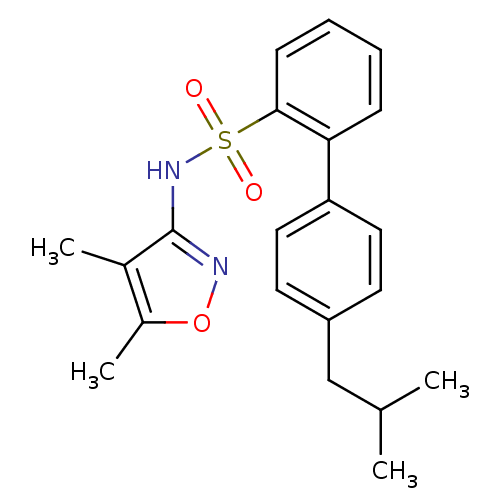 (4'-Isobutyl-biphenyl-2-sulfonic acid (4,5-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068722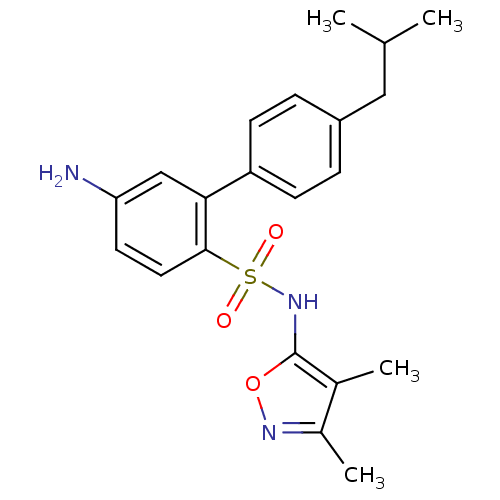 (5-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068704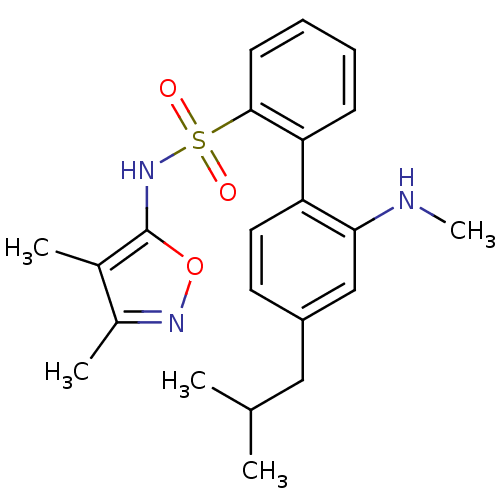 (4'-Isobutyl-2'-methylamino-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068719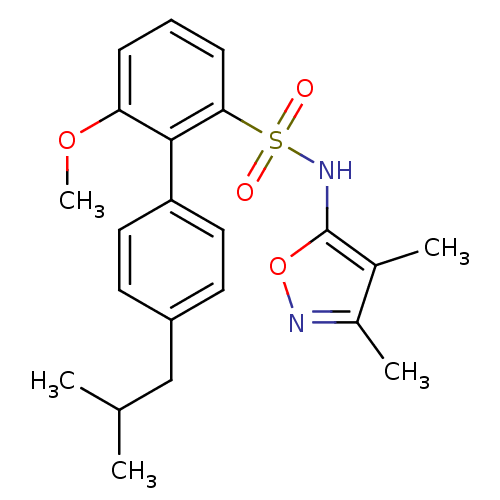 (4'-Isobutyl-6-methoxy-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068716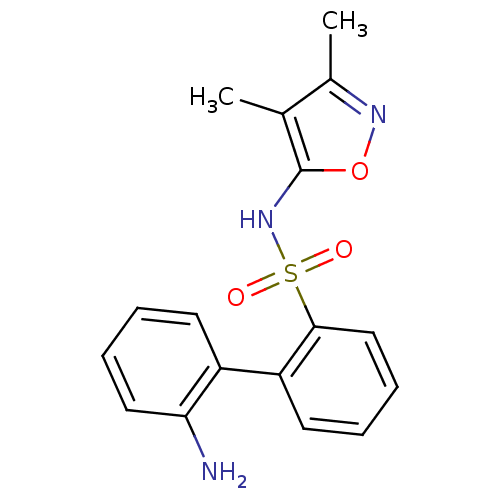 (2'-Amino-biphenyl-2-sulfonic acid (3,4-dimethyl-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068717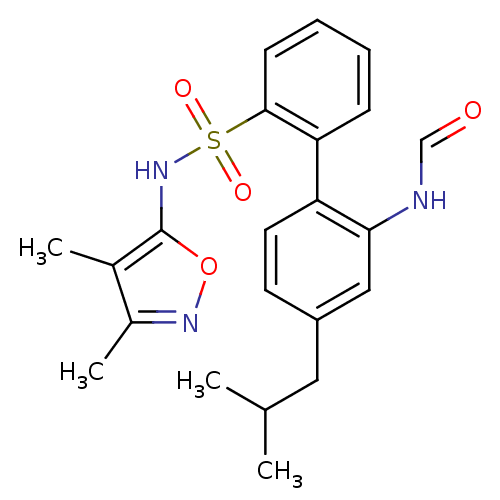 (2'-Formylamino-4'-isobutyl-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068676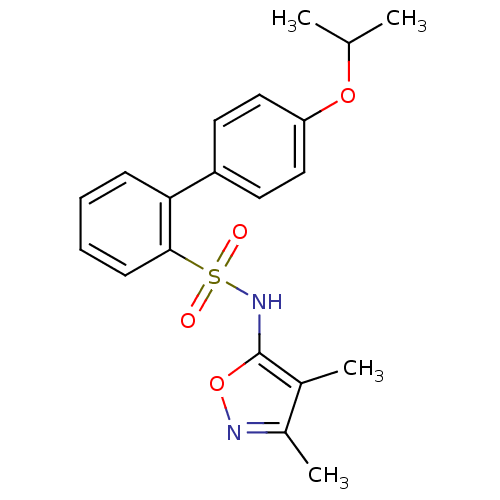 (4'-Isopropoxy-biphenyl-2-sulfonic acid (3,4-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068742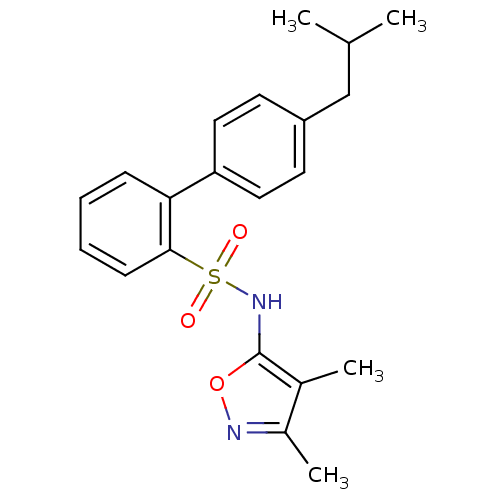 (4'-Isobutyl-biphenyl-2-sulfonic acid (3,4-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068698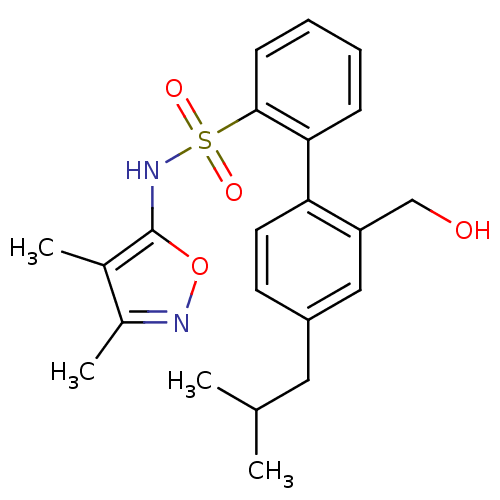 (2'-Hydroxymethyl-4'-isobutyl-biphenyl-2-sulfonic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068729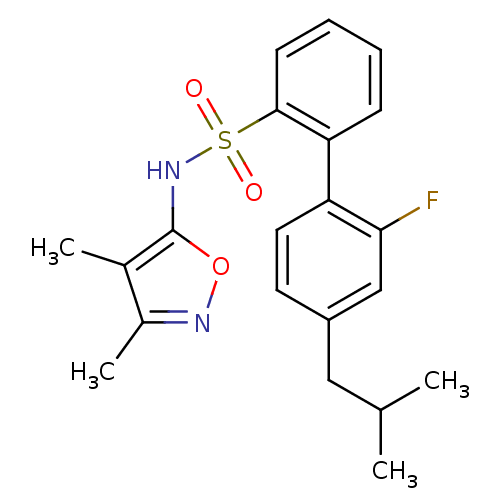 (2'-Fluoro-4'-isobutyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068694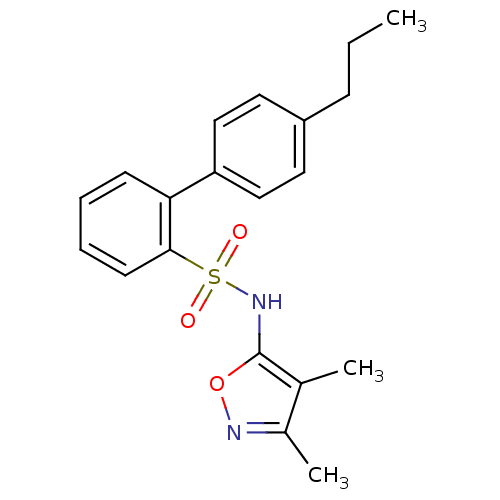 (4'-Propyl-biphenyl-2-sulfonic acid (3,4-dimethyl-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068677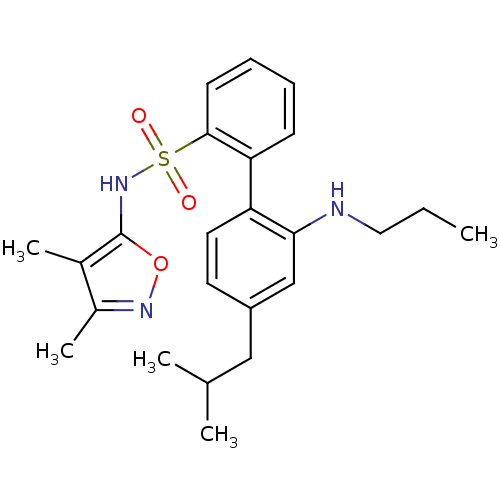 (4'-Isobutyl-2'-propylamino-biphenyl-2-sulfonic aci...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068686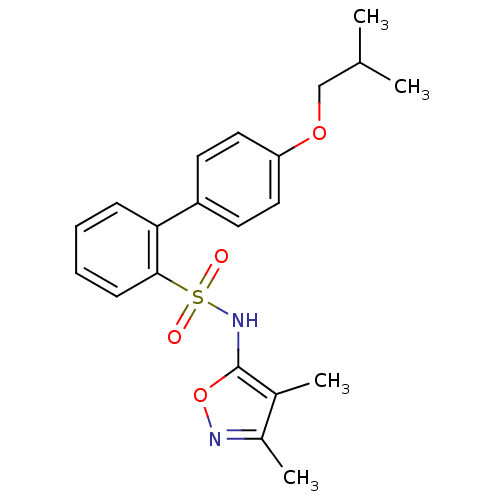 (4'-Isobutoxy-biphenyl-2-sulfonic acid (3,4-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068746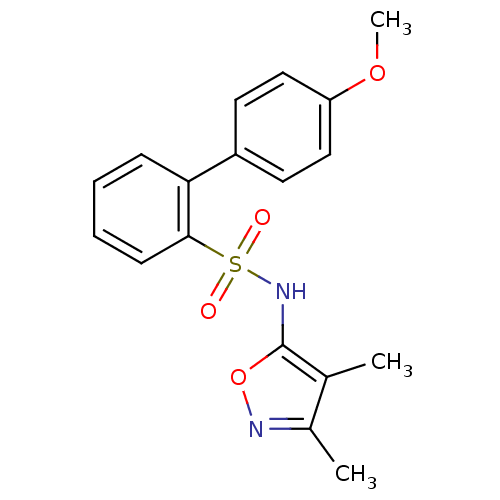 (4'-Methoxy-biphenyl-2-sulfonic acid (3,4-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068738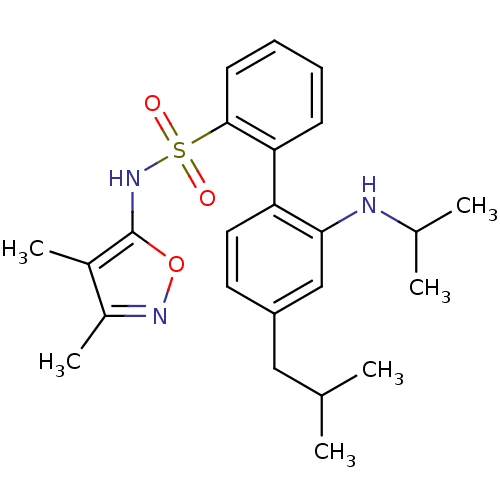 (4'-Isobutyl-2'-isopropylamino-biphenyl-2-sulfonic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068735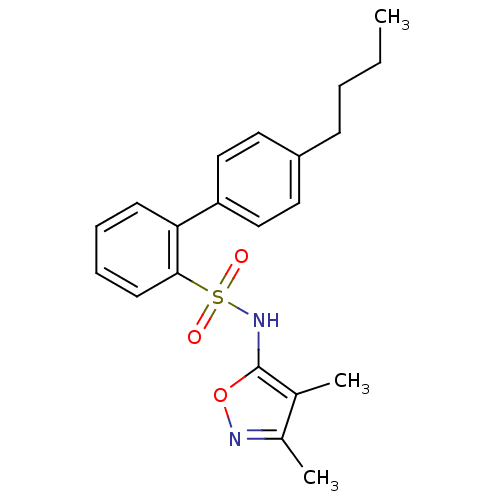 (4'-Butyl-biphenyl-2-sulfonic acid (3,4-dimethyl-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068731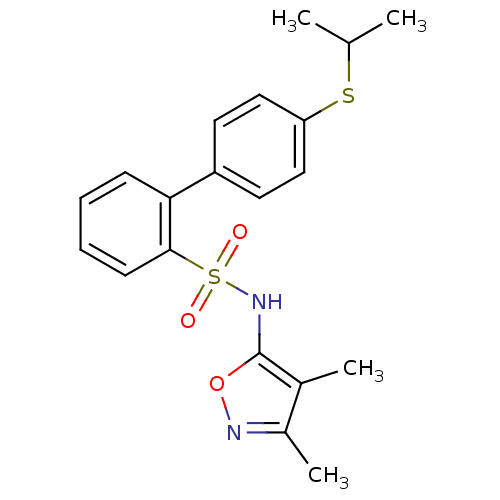 (4'-Isopropylsulfanyl-biphenyl-2-sulfonic acid (3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50329850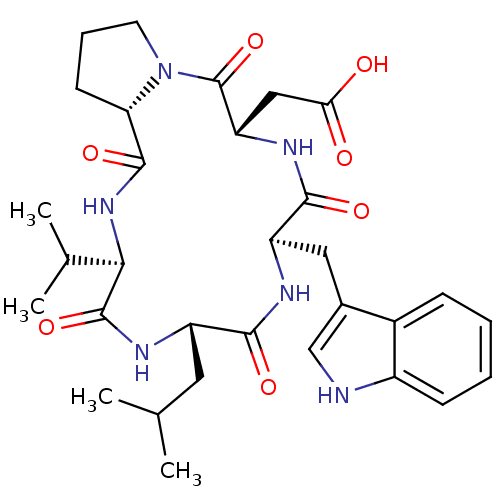 ((R)-3-Amino-4-[(S)-2-((R)-1-{(S)-1-[(R)-1-(1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068703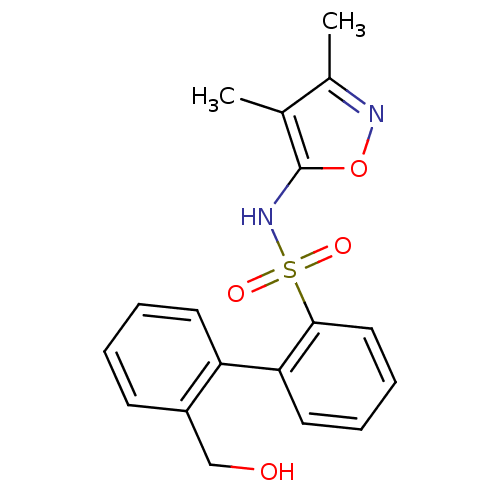 (2'-Hydroxymethyl-biphenyl-2-sulfonic acid (3,4-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068691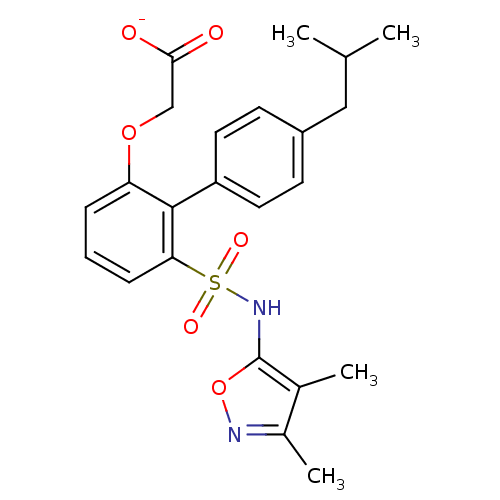 (CHEMBL149152 | Lithium; [6-(3,4-dimethyl-isoxazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068710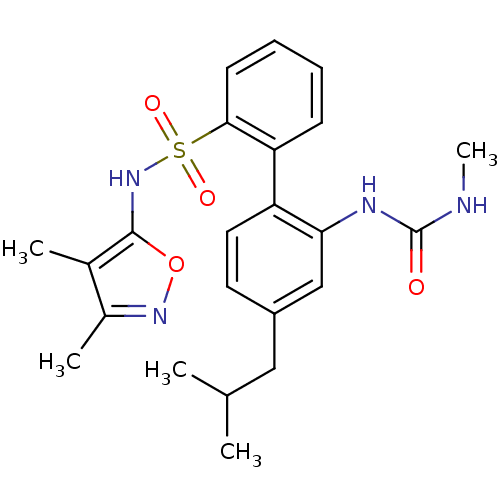 (4'-Isobutyl-2'-(3-methyl-ureido)-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068727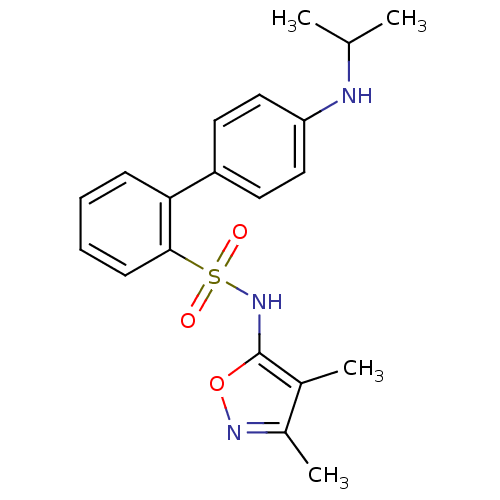 (4'-Isopropylamino-biphenyl-2-sulfonic acid (3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068705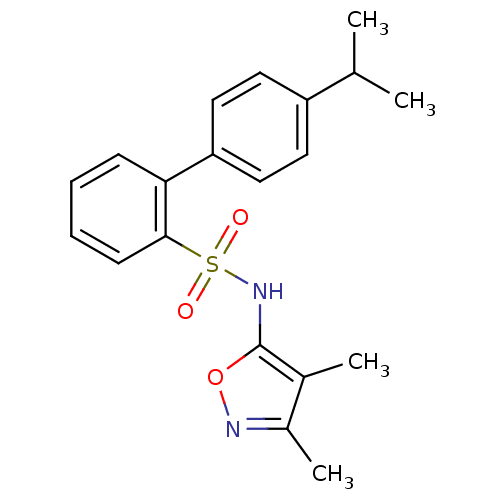 (4'-Isopropyl-biphenyl-2-sulfonic acid (3,4-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068682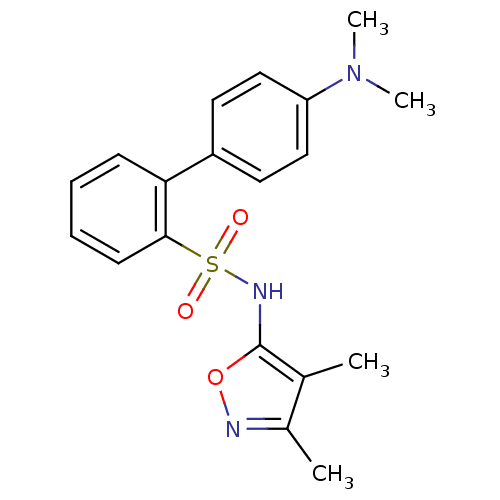 (4'-Dimethylamino-biphenyl-2-sulfonic acid (3,4-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068701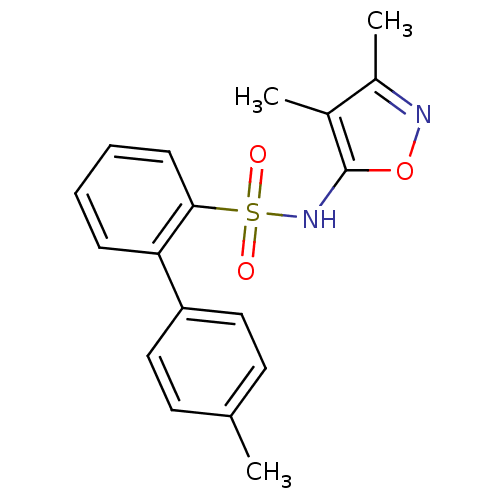 (4'-Methyl-biphenyl-2-sulfonic acid (3,4-dimethyl-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50034435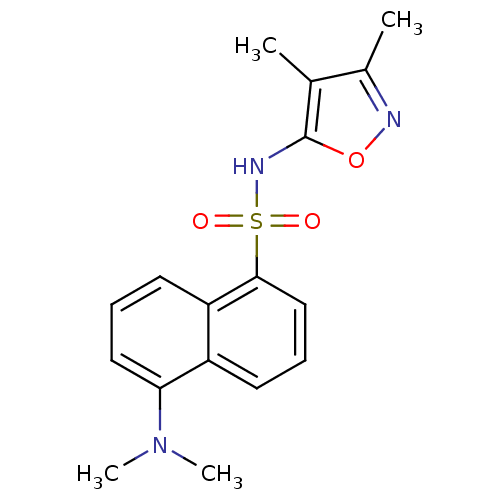 (5-Dimethylamino-naphthalene-1-sulfonic acid (3,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068683 (4'-(Isopropyl-methyl-amino)-biphenyl-2-sulfonic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1028 total ) | Next | Last >> |