

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase FUT5 (Homo sapiens (Human)) | BDBM50366926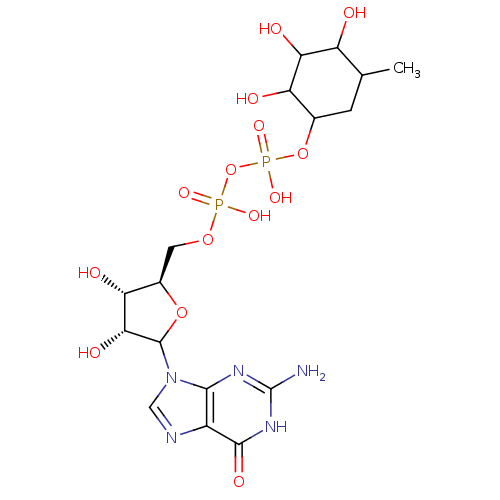 (CHEMBL609638) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
David Geffen School of Medicine at University of California Curated by ChEMBL | Assay Description Inhibition of Fucosyltransferase 5 by the compound was evaluated; Competitive inhibition | Bioorg Med Chem Lett 14: 571-3 (2004) BindingDB Entry DOI: 10.7270/Q2MS3TBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase FUT5 (Homo sapiens (Human)) | BDBM50366925 (CHEMBL610712) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
David Geffen School of Medicine at University of California Curated by ChEMBL | Assay Description Inhibition of Fucosyltransferase 5 by the compound was evaluated; Weak competitive inhibition | Bioorg Med Chem Lett 14: 571-3 (2004) BindingDB Entry DOI: 10.7270/Q2MS3TBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase FUT5 (Homo sapiens (Human)) | BDBM50101904 (CHEMBL52741 | GDP-Azasugar analogue) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of GnRH-stimulated luteinizing hormone (LH) release in rat pituitary cells | Bioorg Med Chem Lett 11: 1809-11 (2001) BindingDB Entry DOI: 10.7270/Q2FF3SWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase FUT5 (Homo sapiens (Human)) | BDBM92459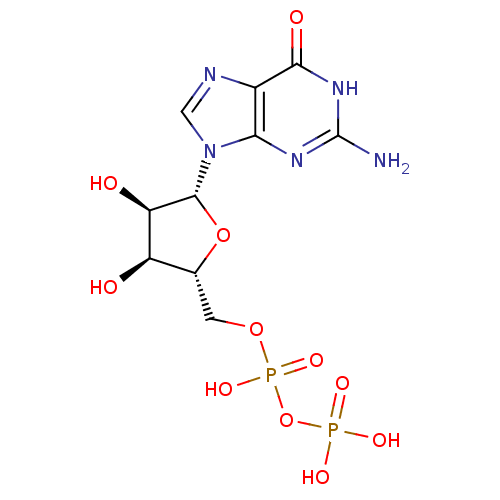 (CHEMBL384759 | GDP | Guanosine Diphosphate) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Berlin Curated by ChEMBL | Assay Description Inhibitory activity against fucosyltransferase (FucT V) in the presence of fucosyl acceptor N-acetyllactosamine | Bioorg Med Chem Lett 11: 1809-11 (2001) BindingDB Entry DOI: 10.7270/Q2FF3SWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase FUT5 (Homo sapiens (Human)) | BDBM50101904 (CHEMBL52741 | GDP-Azasugar analogue) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Berlin Curated by ChEMBL | Assay Description Inhibitory activity against fucosyltransferase (FucT V) in the presence of 10 mM fucosyl acceptor N-acetyllactosamine | Bioorg Med Chem Lett 11: 1809-11 (2001) BindingDB Entry DOI: 10.7270/Q2FF3SWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase FUT5 (Homo sapiens (Human)) | BDBM50101907 (({[(2R,3S,4R,5R)-5-(2-azaniumyl-6-hydroxy-9H-purin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Berlin Curated by ChEMBL | Assay Description Inhibitory activity against fucosyltransferase (FucT V) in the presence of fucosyl acceptor N-acetyllactosamine | Bioorg Med Chem Lett 11: 1809-11 (2001) BindingDB Entry DOI: 10.7270/Q2FF3SWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-galactosyl-N-acetylglucosaminide 3-alpha-L-fucosyltransferase FUT5 (Homo sapiens (Human)) | BDBM50101906 (CHEMBL53188 | GDP-Azasugar analogue) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Berlin Curated by ChEMBL | Assay Description Inhibitory activity against fucosyltransferase (FucT V) in the presence of fucosyl acceptor N-acetyllactosamine | Bioorg Med Chem Lett 11: 1809-11 (2001) BindingDB Entry DOI: 10.7270/Q2FF3SWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||