 Found 839 hits Enz. Inhib. hit(s) with Target = 'Hypoxia-inducible factor 1-alpha' AND taxid = 9606
Found 839 hits Enz. Inhib. hit(s) with Target = 'Hypoxia-inducible factor 1-alpha' AND taxid = 9606 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296480

(CHEMBL553249)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C102H157N25O35/c1-14-51(10)81(101(162)127-34-19-23-73(127)100(161)111-54(13)85(146)117-69(43-77(135)136)95(156)124-72(46-80(141)142)98(159)125-71(45-79(139)140)97(158)122-67(40-55-20-16-15-17-21-55)94(155)114-60(28-30-74(104)130)88(149)119-65(38-49(6)7)91(152)112-59(82(105)143)22-18-33-108-102(106)107)126-99(160)68(41-56-24-26-57(129)27-25-56)121-89(150)62(32-35-128)113-83(144)52(11)110-90(151)63(36-47(2)3)116-84(145)53(12)109-87(148)61(29-31-75(131)132)115-92(153)66(39-50(8)9)120-96(157)70(44-78(137)138)123-93(154)64(37-48(4)5)118-86(147)58(103)42-76(133)134/h15-17,20-21,24-27,47-54,58-73,81,128-129H,14,18-19,22-23,28-46,103H2,1-13H3,(H2,104,130)(H2,105,143)(H,109,148)(H,110,151)(H,111,161)(H,112,152)(H,113,144)(H,114,155)(H,115,153)(H,116,145)(H,117,146)(H,118,147)(H,119,149)(H,120,157)(H,121,150)(H,122,158)(H,123,154)(H,124,156)(H,125,159)(H,126,160)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,141,142)(H4,106,107,108)/t51-,52+,53+,54+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,81+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296473

(CHEMBL558940)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6@@H](-[#8])-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C103H157N25O35/c1-14-51(10)82(102(163)127-33-19-23-73(127)99(160)111-53(12)85(146)117-69(42-78(136)137)94(155)123-72(45-81(142)143)97(158)124-71(44-80(140)141)96(157)121-67(38-55-20-16-15-17-21-55)93(154)114-61(28-30-75(105)131)88(149)119-65(36-49(6)7)90(151)113-60(83(106)144)22-18-32-109-103(107)108)126-98(159)68(39-56-24-26-57(129)27-25-56)125-100(161)74-40-58(130)46-128(74)101(162)54(13)112-89(150)63(34-47(2)3)116-84(145)52(11)110-87(148)62(29-31-76(132)133)115-91(152)66(37-50(8)9)120-95(156)70(43-79(138)139)122-92(153)64(35-48(4)5)118-86(147)59(104)41-77(134)135/h15-17,20-21,24-27,47-54,58-74,82,129-130H,14,18-19,22-23,28-46,104H2,1-13H3,(H2,105,131)(H2,106,144)(H,110,148)(H,111,160)(H,112,150)(H,113,151)(H,114,154)(H,115,152)(H,116,145)(H,117,146)(H,118,147)(H,119,149)(H,120,156)(H,121,157)(H,122,153)(H,123,155)(H,124,158)(H,125,161)(H,126,159)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,107,108,109)/t51-,52+,53+,54+,58-,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,82+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296481

(CHEMBL562618)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6@@H](F)-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C103H156FN25O34/c1-14-51(10)82(102(163)128-33-19-23-73(128)99(160)112-53(12)85(146)118-69(42-78(136)137)94(155)124-72(45-81(142)143)97(158)125-71(44-80(140)141)96(157)122-67(38-55-20-16-15-17-21-55)93(154)115-61(28-30-75(106)131)88(149)120-65(36-49(6)7)90(151)114-60(83(107)144)22-18-32-110-103(108)109)127-98(159)68(39-56-24-26-58(130)27-25-56)126-100(161)74-40-57(104)46-129(74)101(162)54(13)113-89(150)63(34-47(2)3)117-84(145)52(11)111-87(148)62(29-31-76(132)133)116-91(152)66(37-50(8)9)121-95(156)70(43-79(138)139)123-92(153)64(35-48(4)5)119-86(147)59(105)41-77(134)135/h15-17,20-21,24-27,47-54,57,59-74,82,130H,14,18-19,22-23,28-46,105H2,1-13H3,(H2,106,131)(H2,107,144)(H,111,148)(H,112,160)(H,113,150)(H,114,151)(H,115,154)(H,116,152)(H,117,145)(H,118,146)(H,119,147)(H,120,149)(H,121,156)(H,122,157)(H,123,153)(H,124,155)(H,125,158)(H,126,161)(H,127,159)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,108,109,110)/t51-,52+,53+,54+,57-,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,82+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296477

(CHEMBL563460)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C102H156N26O35/c1-14-50(10)81(101(163)128-33-19-23-72(128)100(162)112-53(13)85(147)118-68(42-77(136)137)95(157)125-71(45-80(142)143)98(160)126-70(44-79(140)141)97(159)122-65(38-54-20-16-15-17-21-54)93(155)114-59(28-30-73(104)130)88(150)120-63(36-48(6)7)90(152)113-58(82(106)144)22-18-32-109-102(107)108)127-99(161)66(39-55-24-26-56(129)27-25-55)123-94(156)67(41-74(105)131)117-84(146)52(12)111-89(151)61(34-46(2)3)116-83(145)51(11)110-87(149)60(29-31-75(132)133)115-91(153)64(37-49(8)9)121-96(158)69(43-78(138)139)124-92(154)62(35-47(4)5)119-86(148)57(103)40-76(134)135/h15-17,20-21,24-27,46-53,57-72,81,129H,14,18-19,22-23,28-45,103H2,1-13H3,(H2,104,130)(H2,105,131)(H2,106,144)(H,110,149)(H,111,151)(H,112,162)(H,113,152)(H,114,155)(H,115,153)(H,116,145)(H,117,146)(H,118,147)(H,119,148)(H,120,150)(H,121,158)(H,122,159)(H,123,156)(H,124,154)(H,125,157)(H,126,160)(H,127,161)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,107,108,109)/t50-,51+,52+,53+,57+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,81+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296478

(CHEMBL557433)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C102H155N25O36/c1-14-50(10)81(101(163)127-33-19-23-72(127)100(162)111-53(13)85(147)117-68(42-77(136)137)95(157)124-71(45-80(142)143)98(160)125-70(44-79(140)141)97(159)121-65(38-54-20-16-15-17-21-54)93(155)113-59(28-30-73(104)129)88(150)119-63(36-48(6)7)90(152)112-58(82(105)144)22-18-32-108-102(106)107)126-99(161)66(39-55-24-26-56(128)27-25-55)122-94(156)67(41-76(134)135)116-84(146)52(12)110-89(151)61(34-46(2)3)115-83(145)51(11)109-87(149)60(29-31-74(130)131)114-91(153)64(37-49(8)9)120-96(158)69(43-78(138)139)123-92(154)62(35-47(4)5)118-86(148)57(103)40-75(132)133/h15-17,20-21,24-27,46-53,57-72,81,128H,14,18-19,22-23,28-45,103H2,1-13H3,(H2,104,129)(H2,105,144)(H,109,149)(H,110,151)(H,111,162)(H,112,152)(H,113,155)(H,114,153)(H,115,145)(H,116,146)(H,117,147)(H,118,148)(H,119,150)(H,120,158)(H,121,159)(H,122,156)(H,123,154)(H,124,157)(H,125,160)(H,126,161)(H,130,131)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,106,107,108)/t50-,51+,52+,53+,57+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,81+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296476
(CHEMBL557420)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C103H159N25O34/c1-16-52(12)82(102(162)128-35-21-25-73(128)100(160)112-54(14)85(145)117-69(43-77(135)136)95(155)123-72(46-80(141)142)98(158)124-71(45-79(139)140)97(157)121-67(40-56-22-18-17-19-23-56)94(154)114-61(30-32-74(105)130)89(149)119-65(38-49(6)7)91(151)113-60(83(106)143)24-20-34-109-103(107)108)127-99(159)68(41-57-26-28-58(129)29-27-57)125-101(161)81(51(10)11)126-86(146)55(15)111-90(150)63(36-47(2)3)116-84(144)53(13)110-88(148)62(31-33-75(131)132)115-92(152)66(39-50(8)9)120-96(156)70(44-78(137)138)122-93(153)64(37-48(4)5)118-87(147)59(104)42-76(133)134/h17-19,22-23,26-29,47-55,59-73,81-82,129H,16,20-21,24-25,30-46,104H2,1-15H3,(H2,105,130)(H2,106,143)(H,110,148)(H,111,150)(H,112,160)(H,113,151)(H,114,154)(H,115,152)(H,116,144)(H,117,145)(H,118,147)(H,119,149)(H,120,156)(H,121,157)(H,122,153)(H,123,155)(H,124,158)(H,125,161)(H,126,146)(H,127,159)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,141,142)(H4,107,108,109)/t52-,53+,54+,55+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,81+,82+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296479
(CHEMBL562806)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6@H](-[#6])-[#8])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C102H157N25O35/c1-15-50(10)80(101(162)127-34-20-24-72(127)99(160)111-52(12)84(145)116-68(42-76(135)136)94(155)122-71(45-79(141)142)97(158)123-70(44-78(139)140)96(157)120-66(39-55-21-17-16-18-22-55)93(154)113-60(29-31-73(104)130)88(149)118-64(37-48(6)7)90(151)112-59(82(105)143)23-19-33-108-102(106)107)125-98(159)67(40-56-25-27-57(129)28-26-56)124-100(161)81(54(14)128)126-85(146)53(13)110-89(150)62(35-46(2)3)115-83(144)51(11)109-87(148)61(30-32-74(131)132)114-91(152)65(38-49(8)9)119-95(156)69(43-77(137)138)121-92(153)63(36-47(4)5)117-86(147)58(103)41-75(133)134/h16-18,21-22,25-28,46-54,58-72,80-81,128-129H,15,19-20,23-24,29-45,103H2,1-14H3,(H2,104,130)(H2,105,143)(H,109,148)(H,110,150)(H,111,160)(H,112,151)(H,113,154)(H,114,152)(H,115,144)(H,116,145)(H,117,147)(H,118,149)(H,119,156)(H,120,157)(H,121,153)(H,122,155)(H,123,158)(H,124,161)(H,125,159)(H,126,146)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,141,142)(H4,106,107,108)/t50-,51+,52+,53+,54+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,80+,81+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296475
(CHEMBL557344)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C104H161N25O34/c1-16-52(11)82(127-87(147)56(15)112-91(151)64(37-48(3)4)117-85(145)54(13)111-89(149)63(32-34-76(132)133)116-93(153)67(40-51(9)10)121-97(157)71(45-79(138)139)123-94(154)65(38-49(5)6)119-88(148)60(105)43-77(134)135)102(162)126-69(42-58-27-29-59(130)30-28-58)100(160)128-83(53(12)17-2)103(163)129-36-22-26-74(129)101(161)113-55(14)86(146)118-70(44-78(136)137)96(156)124-73(47-81(142)143)99(159)125-72(46-80(140)141)98(158)122-68(41-57-23-19-18-20-24-57)95(155)115-62(31-33-75(106)131)90(150)120-66(39-50(7)8)92(152)114-61(84(107)144)25-21-35-110-104(108)109/h18-20,23-24,27-30,48-56,60-74,82-83,130H,16-17,21-22,25-26,31-47,105H2,1-15H3,(H2,106,131)(H2,107,144)(H,111,149)(H,112,151)(H,113,161)(H,114,152)(H,115,155)(H,116,153)(H,117,145)(H,118,146)(H,119,148)(H,120,150)(H,121,157)(H,122,158)(H,123,154)(H,124,156)(H,125,159)(H,126,162)(H,127,147)(H,128,160)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,108,109,110)/t52-,53-,54+,55+,56+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,82+,83+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296474

(CHEMBL558009)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C104H161N25O34/c1-16-53(12)83(103(163)129-35-21-25-75(129)102(162)113-56(15)87(147)119-71(44-79(136)137)97(157)126-74(47-82(142)143)100(160)127-73(46-81(140)141)99(159)123-69(41-57-22-18-17-19-23-57)96(156)115-62(30-32-76(106)131)90(150)121-67(39-51(8)9)92(152)114-61(84(107)144)24-20-34-110-104(108)109)128-101(161)70(42-58-26-28-59(130)29-27-58)124-94(154)65(37-49(4)5)118-86(146)55(14)112-91(151)64(36-48(2)3)117-85(145)54(13)111-89(149)63(31-33-77(132)133)116-93(153)68(40-52(10)11)122-98(158)72(45-80(138)139)125-95(155)66(38-50(6)7)120-88(148)60(105)43-78(134)135/h17-19,22-23,26-29,48-56,60-75,83,130H,16,20-21,24-25,30-47,105H2,1-15H3,(H2,106,131)(H2,107,144)(H,111,149)(H,112,151)(H,113,162)(H,114,152)(H,115,156)(H,116,153)(H,117,145)(H,118,146)(H,119,147)(H,120,148)(H,121,150)(H,122,158)(H,123,159)(H,124,154)(H,125,155)(H,126,157)(H,127,160)(H,128,161)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,108,109,110)/t53-,54+,55+,56+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,75+,83+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296482

(CHEMBL541769)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C104H159N25O34/c1-14-53(10)83(103(163)129-37-21-26-75(129)100(160)112-55(12)86(146)118-70(45-79(136)137)95(155)124-73(48-82(142)143)98(158)125-72(47-81(140)141)97(157)122-68(42-57-22-16-15-17-23-57)94(154)115-62(31-33-76(106)131)89(149)120-66(40-51(6)7)91(151)114-61(84(107)144)24-20-35-110-104(108)109)127-99(159)69(43-58-27-29-59(130)30-28-58)126-101(161)74-25-18-19-36-128(74)102(162)56(13)113-90(150)64(38-49(2)3)117-85(145)54(11)111-88(148)63(32-34-77(132)133)116-92(152)67(41-52(8)9)121-96(156)71(46-80(138)139)123-93(153)65(39-50(4)5)119-87(147)60(105)44-78(134)135/h15-17,22-23,27-30,49-56,60-75,83,130H,14,18-21,24-26,31-48,105H2,1-13H3,(H2,106,131)(H2,107,144)(H,111,148)(H,112,160)(H,113,150)(H,114,151)(H,115,154)(H,116,152)(H,117,145)(H,118,146)(H,119,147)(H,120,149)(H,121,156)(H,122,157)(H,123,153)(H,124,155)(H,125,158)(H,126,161)(H,127,159)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,108,109,110)/t53-,54+,55+,56+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,75+,83+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha inhibitor
(Homo sapiens (Human)) | BDBM26108
((2R)-2-(formamidoformic acid)-4-phenylbutanoic aci...)Show InChI InChI=1S/C12H13NO5/c14-10(12(17)18)13-9(11(15)16)7-6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,13,14)(H,15,16)(H,17,18)/t9-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of asparaginyl hydroxylase factor-inhibiting-HIF |
Bioorg Med Chem 19: 3625-36 (2011)
Article DOI: 10.1016/j.bmc.2011.01.046
BindingDB Entry DOI: 10.7270/Q23X870S |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296483
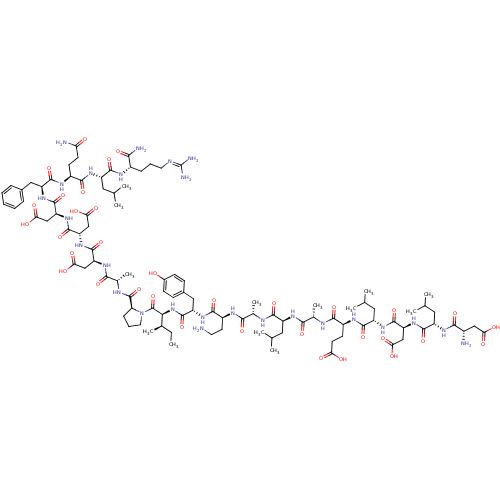
(CHEMBL538372)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C102H158N26O34/c1-14-51(10)81(101(162)128-35-19-23-73(128)100(161)112-54(13)85(146)118-69(43-77(135)136)95(156)125-72(46-80(141)142)98(159)126-71(45-79(139)140)97(158)123-67(40-55-20-16-15-17-21-55)94(155)115-60(28-30-74(105)130)88(149)120-65(38-49(6)7)91(152)113-59(82(106)143)22-18-34-109-102(107)108)127-99(160)68(41-56-24-26-57(129)27-25-56)122-89(150)62(32-33-103)114-83(144)52(11)111-90(151)63(36-47(2)3)117-84(145)53(12)110-87(148)61(29-31-75(131)132)116-92(153)66(39-50(8)9)121-96(157)70(44-78(137)138)124-93(154)64(37-48(4)5)119-86(147)58(104)42-76(133)134/h15-17,20-21,24-27,47-54,58-73,81,129H,14,18-19,22-23,28-46,103-104H2,1-13H3,(H2,105,130)(H2,106,143)(H,110,148)(H,111,151)(H,112,161)(H,113,152)(H,114,144)(H,115,155)(H,116,153)(H,117,145)(H,118,146)(H,119,147)(H,120,149)(H,121,157)(H,122,150)(H,123,158)(H,124,154)(H,125,156)(H,126,159)(H,127,160)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,141,142)(H4,107,108,109)/t51-,52+,53+,54+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,81+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296472
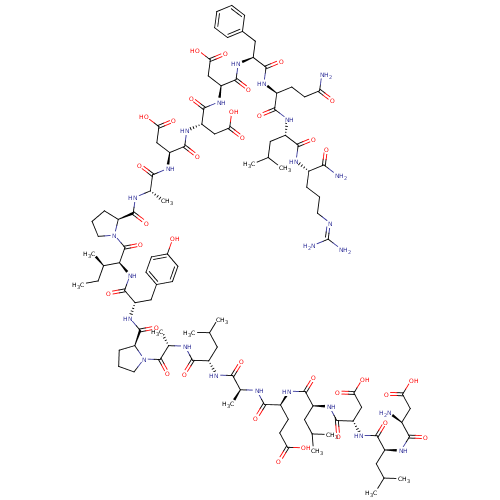
(CHEMBL558824)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C103H157N25O34/c1-14-52(10)82(102(162)128-36-20-24-73(128)99(159)111-54(12)85(145)117-69(44-78(135)136)94(154)123-72(47-81(141)142)97(157)124-71(46-80(139)140)96(156)121-67(41-56-21-16-15-17-22-56)93(153)114-61(30-32-75(105)130)88(148)119-65(39-50(6)7)90(150)113-60(83(106)143)23-18-34-109-103(107)108)126-98(158)68(42-57-26-28-58(129)29-27-57)125-100(160)74-25-19-35-127(74)101(161)55(13)112-89(149)63(37-48(2)3)116-84(144)53(11)110-87(147)62(31-33-76(131)132)115-91(151)66(40-51(8)9)120-95(155)70(45-79(137)138)122-92(152)64(38-49(4)5)118-86(146)59(104)43-77(133)134/h15-17,21-22,26-29,48-55,59-74,82,129H,14,18-20,23-25,30-47,104H2,1-13H3,(H2,105,130)(H2,106,143)(H,110,147)(H,111,159)(H,112,149)(H,113,150)(H,114,153)(H,115,151)(H,116,144)(H,117,145)(H,118,146)(H,119,148)(H,120,155)(H,121,156)(H,122,152)(H,123,154)(H,124,157)(H,125,160)(H,126,158)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,141,142)(H4,107,108,109)/t52-,53+,54+,55+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,82+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha inhibitor
(Homo sapiens (Human)) | BDBM26106
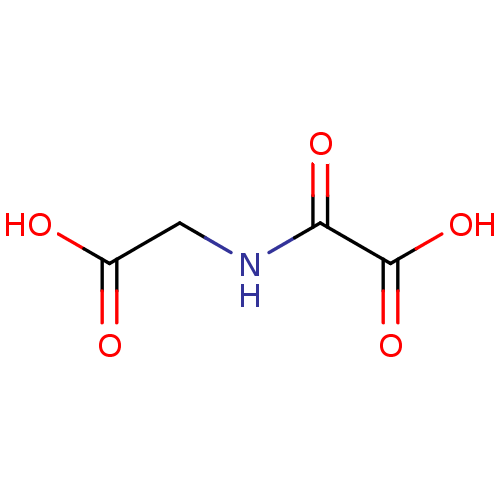
(CHEMBL90852 | N-oxalyl glycine, 1a | NOG | Oxalylg...)Show InChI InChI=1S/C4H5NO5/c6-2(7)1-5-3(8)4(9)10/h1H2,(H,5,8)(H,6,7)(H,9,10) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Inhibition of asparaginyl hydroxylase factor-inhibiting-HIF |
Bioorg Med Chem 19: 3625-36 (2011)
Article DOI: 10.1016/j.bmc.2011.01.046
BindingDB Entry DOI: 10.7270/Q23X870S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50179013
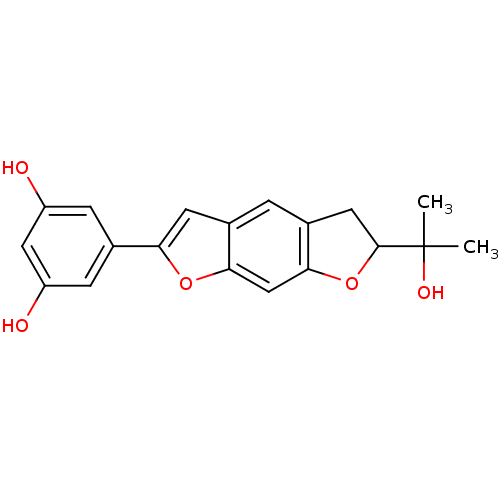
(CHEMBL205924 | moracin O)Show SMILES CC(C)(O)C1Cc2cc3cc(oc3cc2O1)-c1cc(O)cc(O)c1 Show InChI InChI=1S/C19H18O5/c1-19(2,22)18-7-11-3-10-6-15(23-16(10)9-17(11)24-18)12-4-13(20)8-14(21)5-12/h3-6,8-9,18,20-22H,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Research Institute of Biosciences and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha protein accumulation in human Hep3B cells treated for 30 mins measured after 12 hrs by Western blot analysis |
J Nat Prod 72: 39-43 (2009)
Article DOI: 10.1021/np800491u
BindingDB Entry DOI: 10.7270/Q27947GK |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50179011
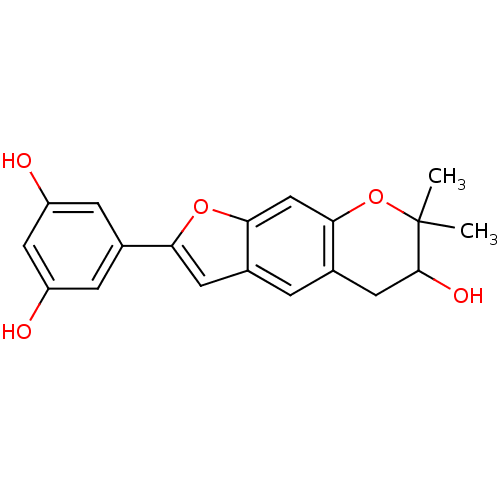
(CHEMBL380456 | Moracin P)Show InChI InChI=1S/C19H18O5/c1-19(2)18(22)7-11-3-10-6-15(23-16(10)9-17(11)24-19)12-4-13(20)8-14(21)5-12/h3-6,8-9,18,20-22H,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus FabI-mediated trans-2-octenoyl N-acetylcysteamine (t-o-NAC thioester) substrate reduction assessed as decrease in... |
Eur J Med Chem 90: 379-93 (2015)
Article DOI: 10.1016/j.ejmech.2014.11.047
BindingDB Entry DOI: 10.7270/Q2GF0W5F |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50480476

((-)-Moracin P | Moracin P)Show SMILES CC1(C)Oc2cc3oc(cc3cc2C[C@H]1O)-c1cc(O)cc(O)c1 |r| Show InChI InChI=1S/C19H18O5/c1-19(2)18(22)7-11-3-10-6-15(23-16(10)9-17(11)24-19)12-4-13(20)8-14(21)5-12/h3-6,8-9,18,20-22H,7H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Research Institute of Biosciences and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha protein accumulation in human Hep3B cells treated for 30 mins measured after 12 hrs by Western blot analysis |
J Nat Prod 72: 39-43 (2009)
Article DOI: 10.1021/np800491u
BindingDB Entry DOI: 10.7270/Q27947GK |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50346783
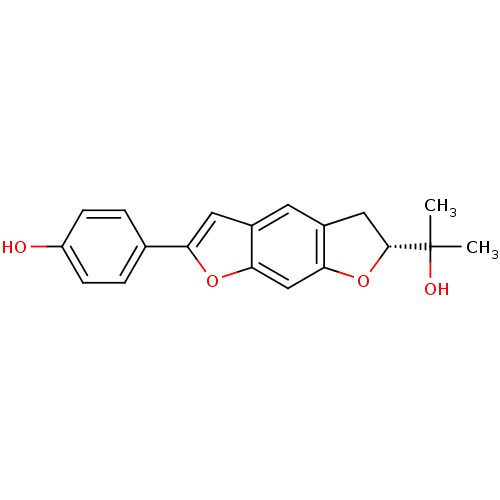
(CHEMBL1795446)Show SMILES CC(C)(O)[C@H]1Cc2cc3cc(oc3cc2O1)-c1ccc(O)cc1 |r| Show InChI InChI=1S/C19H18O4/c1-19(2,21)18-9-13-7-12-8-15(11-3-5-14(20)6-4-11)22-16(12)10-17(13)23-18/h3-8,10,18,20-21H,9H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay |
Eur J Med Chem 46: 2386-96 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.022
BindingDB Entry DOI: 10.7270/Q2WD40XF |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50524833

(CHEMBL283631 | NSC-628679)Show SMILES [H][C@]12COC(=O)[C@]1([H])[C@H](c1cc(OC)c(O)c(OC)c1)c1cc3OCOc3cc1[C@H]2Nc1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C27H24N2O9/c1-34-21-7-13(8-22(35-2)26(21)30)23-16-9-19-20(38-12-37-19)10-17(16)25(18-11-36-27(31)24(18)23)28-14-3-5-15(6-4-14)29(32)33/h3-10,18,23-25,28,30H,11-12H2,1-2H3/t18-,23+,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1alpha in human U373 cells |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50401029

(CHEMBL2205734)Show SMILES Cc1cc(nn1Cc1ccc(C)cc1)-c1nc2cc(ccc2[nH]1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H21ClN4/c1-16-3-5-18(6-4-16)15-30-17(2)13-24(29-30)25-27-22-12-9-20(14-23(22)28-25)19-7-10-21(26)11-8-19/h3-14H,15H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1alpha in human HCT116 cells under hypoxia condition by HRE-luciferase reporter gene assay |
Eur J Med Chem 49: 24-40 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.033
BindingDB Entry DOI: 10.7270/Q26D5V4N |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50511097

(CHEMBL4538400)Show SMILES COc1ccc(cc1OC)[C@@H](O)[C@@H](C)Oc1ccc(cc1OC)[C@H]1CC[C@H](C1)c1ccc(O[C@H](C)[C@H](O)c2ccc(OC)c(OC)c2)c(OC)c1 |r| Show InChI InChI=1S/C41H50O10/c1-24(40(42)30-13-15-32(44-3)36(22-30)46-5)50-34-17-11-28(20-38(34)48-7)26-9-10-27(19-26)29-12-18-35(39(21-29)49-8)51-25(2)41(43)31-14-16-33(45-4)37(23-31)47-6/h11-18,20-27,40-43H,9-10,19H2,1-8H3/t24-,25-,26-,27+,40+,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Activation of HIF1 in human T47D cells after 30 mins by luciferase reporter gene assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115235
BindingDB Entry DOI: 10.7270/Q2XK8JW5 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50346783

(CHEMBL1795446)Show SMILES CC(C)(O)[C@H]1Cc2cc3cc(oc3cc2O1)-c1ccc(O)cc1 |r| Show InChI InChI=1S/C19H18O4/c1-19(2,21)18-9-13-7-12-8-15(11-3-5-14(20)6-4-11)22-16(12)10-17(13)23-18/h3-8,10,18,20-21H,9H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay |
Eur J Med Chem 46: 2386-96 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.022
BindingDB Entry DOI: 10.7270/Q2WD40XF |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50179013

(CHEMBL205924 | moracin O)Show SMILES CC(C)(O)C1Cc2cc3cc(oc3cc2O1)-c1cc(O)cc(O)c1 Show InChI InChI=1S/C19H18O5/c1-19(2,22)18-7-11-3-10-6-15(23-16(10)9-17(11)24-18)12-4-13(20)8-14(21)5-12/h3-6,8-9,18,20-22H,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay |
Eur J Med Chem 46: 2386-96 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.022
BindingDB Entry DOI: 10.7270/Q2WD40XF |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50346796

(CHEMBL1795432)Show SMILES CC(C)(O)[C@H]1Cc2cc3cc(oc3cc2O1)-c1cc(O)cc(O)c1 |r| Show InChI InChI=1S/C19H18O5/c1-19(2,22)18-7-11-3-10-6-15(23-16(10)9-17(11)24-18)12-4-13(20)8-14(21)5-12/h3-6,8-9,18,20-22H,7H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay |
Eur J Med Chem 46: 2386-96 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.022
BindingDB Entry DOI: 10.7270/Q2WD40XF |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50346790

(CHEMBL1795439)Show InChI InChI=1S/C20H20O4/c1-20(2,21)19-10-14-7-13-9-16(23-17(13)11-18(14)24-19)12-5-4-6-15(8-12)22-3/h4-9,11,19,21H,10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay |
Eur J Med Chem 46: 2386-96 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.022
BindingDB Entry DOI: 10.7270/Q2WD40XF |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50346795
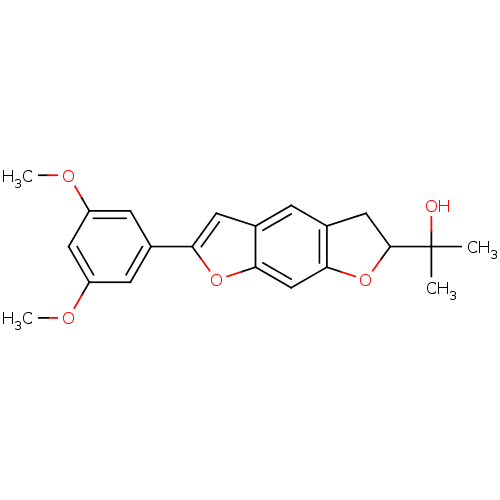
(CHEMBL1795433)Show SMILES COc1cc(OC)cc(c1)-c1cc2cc3CC(Oc3cc2o1)C(C)(C)O Show InChI InChI=1S/C21H22O5/c1-21(2,22)20-9-13-5-12-8-17(25-18(12)11-19(13)26-20)14-6-15(23-3)10-16(7-14)24-4/h5-8,10-11,20,22H,9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay |
Eur J Med Chem 46: 2386-96 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.022
BindingDB Entry DOI: 10.7270/Q2WD40XF |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50179011

(CHEMBL380456 | Moracin P)Show InChI InChI=1S/C19H18O5/c1-19(2)18(22)7-11-3-10-6-15(23-16(10)9-17(11)24-19)12-4-13(20)8-14(21)5-12/h3-6,8-9,18,20-22H,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay |
Eur J Med Chem 46: 2386-96 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.022
BindingDB Entry DOI: 10.7270/Q2WD40XF |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50426548
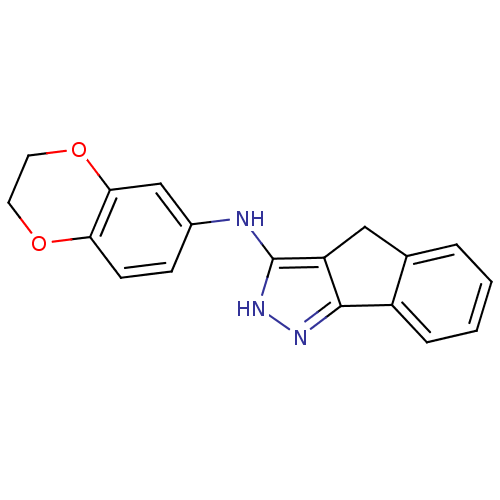
(CHEMBL2323956)Show InChI InChI=1S/C18H15N3O2/c1-2-4-13-11(3-1)9-14-17(13)20-21-18(14)19-12-5-6-15-16(10-12)23-8-7-22-15/h1-6,10H,7-9H2,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Gakushuin University
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha transcriptional activity in human HeLa cells expressing HRE-Luc after 12 hrs by luciferase reporter gene assa... |
ACS Med Chem Lett 4: 297-301 (2013)
Article DOI: 10.1021/ml3004632
BindingDB Entry DOI: 10.7270/Q2R212P9 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50498797

(CHEMBL3105545)Show SMILES COc1ccc(cc1OC)[C@@H](O)[C@@H](C)Oc1ccc(cc1OC)[C@H]1O[C@@H]([C@H](C)[C@H]1C)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C32H40O8/c1-18-19(2)32(40-31(18)22-10-13-25(35-5)28(16-22)37-7)23-11-14-26(29(17-23)38-8)39-20(3)30(33)21-9-12-24(34-4)27(15-21)36-6/h9-20,30-33H,1-8H3/t18-,19-,20-,30+,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Activation of HIF1 in human T47D cells |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115235
BindingDB Entry DOI: 10.7270/Q2XK8JW5 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50511099

(CHEMBL4434875)Show SMILES COc1ccc(cc1OC)[C@@H](O)[C@@H](C)Oc1ccc(cc1OC)[C@@H]1CCN(C1)c1ccc(OC)c(OC)c1 |r| Show InChI InChI=1S/C30H37NO7/c1-19(30(32)21-8-10-24(33-2)27(16-21)35-4)38-26-11-7-20(15-28(26)36-5)22-13-14-31(18-22)23-9-12-25(34-3)29(17-23)37-6/h7-12,15-17,19,22,30,32H,13-14,18H2,1-6H3/t19-,22-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Activation of HIF1 in human T47D cells |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115235
BindingDB Entry DOI: 10.7270/Q2XK8JW5 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50593328

(CHEMBL5205360) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00308
BindingDB Entry DOI: 10.7270/Q2G73JR0 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50593342
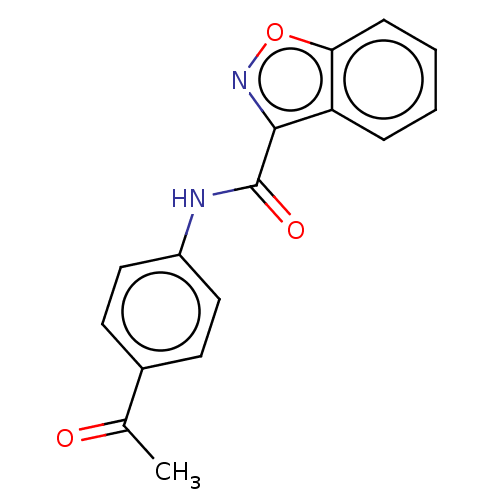
(CHEMBL5184354) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00308
BindingDB Entry DOI: 10.7270/Q2G73JR0 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50346784

(CHEMBL1795445)Show InChI InChI=1S/C17H18N2O3/c1-17(2,20)16-6-11-4-10-5-15(12-8-18-9-19(12)3)21-13(10)7-14(11)22-16/h4-5,7-9,16,20H,6H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay |
Eur J Med Chem 46: 2386-96 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.022
BindingDB Entry DOI: 10.7270/Q2WD40XF |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50426547

(CHEMBL2323957)Show InChI InChI=1S/C19H17N3O3/c1-23-15-4-2-3-12-13(15)10-14-18(12)21-22-19(14)20-11-5-6-16-17(9-11)25-8-7-24-16/h2-6,9H,7-8,10H2,1H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Gakushuin University
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha transcriptional activity in human HeLa cells expressing HRE-Luc after 12 hrs by luciferase reporter gene assa... |
ACS Med Chem Lett 4: 297-301 (2013)
Article DOI: 10.1021/ml3004632
BindingDB Entry DOI: 10.7270/Q2R212P9 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50593331

(CHEMBL5208153) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00308
BindingDB Entry DOI: 10.7270/Q2G73JR0 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50315537
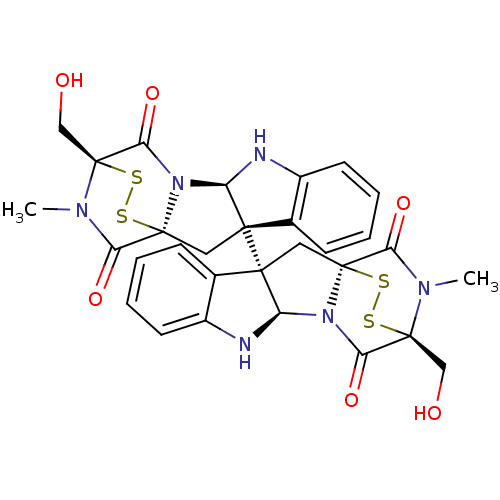
(CHEMBL1089316 | chaetocin)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(CO)SS3)[C@]12C[C@]34SS[C@](CO)(N(C)C3=O)C(=O)N4[C@H]1Nc1ccccc21 |r,THB:36:35:31.33:26.27,7:15:1.2:22.21,38:37:31.33:26.27,17:16:1.2:22.21| Show InChI InChI=1S/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40)34(2)30(14-38,46-44-28)24(42)36(28)20(26)32-18-10-6-4-8-16(18)26/h3-10,19-20,31-32,37-38H,11-14H2,1-2H3/t19-,20-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1alpha-mediated VEGF expression in human Hep3b cells by luciferase reporter gene assay |
Eur J Med Chem 49: 24-40 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.033
BindingDB Entry DOI: 10.7270/Q26D5V4N |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50593334

(CHEMBL5194403) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00308
BindingDB Entry DOI: 10.7270/Q2G73JR0 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50593339

(CHEMBL5197796) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00308
BindingDB Entry DOI: 10.7270/Q2G73JR0 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50396169

(CHEMBL1337170)Show InChI InChI=1S/C8H5NO4S/c10-9(11)7-2-1-6-3-4-14(12,13)8(6)5-7/h1-5H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to Cys255 residue of C-terminal Avi/FLAG-tagged HIF-1alpha (unknown origin) after 1 hr by AlphaScreen assay |
Bioorg Med Chem 27: 1145-1158 (2019)
Article DOI: 10.1016/j.bmc.2019.01.042
BindingDB Entry DOI: 10.7270/Q2QC072Q |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50346794

(CHEMBL1795434)Show InChI InChI=1S/C19H18O4/c1-19(2,21)18-9-13-6-12-8-15(11-4-3-5-14(20)7-11)22-16(12)10-17(13)23-18/h3-8,10,18,20-21H,9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay |
Eur J Med Chem 46: 2386-96 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.022
BindingDB Entry DOI: 10.7270/Q2WD40XF |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50576956

(CHEMBL4849804)Show SMILES [H][C@@]12C[C@@](C)(CC[C@]1(C)CC[C@]1(C)C3=CC=C4C(C)=C(O)C(=O)C=C4[C@]3(C)CC[C@@]21C)C(=O)OCCOc1cnc2ccccc2c1 |r,c:24,t:14,16,19| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hypoxia-induced HIF1alpha transcriptional activity in human Hep3B cells co-transfected with luciferase reporter plasmid containing six ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113474
BindingDB Entry DOI: 10.7270/Q2WQ07MW |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50346793
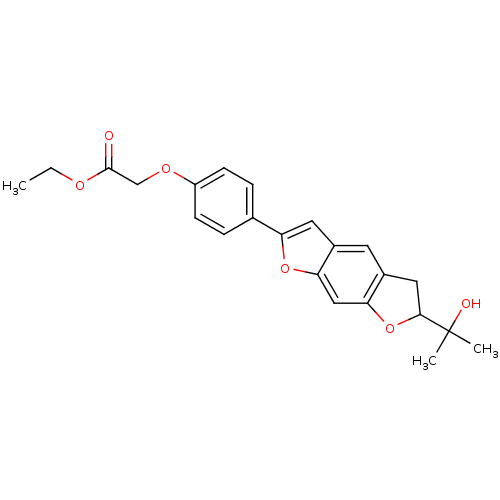
(CHEMBL1795436)Show SMILES CCOC(=O)COc1ccc(cc1)-c1cc2cc3CC(Oc3cc2o1)C(C)(C)O Show InChI InChI=1S/C23H24O6/c1-4-26-22(24)13-27-17-7-5-14(6-8-17)18-10-15-9-16-11-21(23(2,3)25)29-20(16)12-19(15)28-18/h5-10,12,21,25H,4,11,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay |
Eur J Med Chem 46: 2386-96 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.022
BindingDB Entry DOI: 10.7270/Q2WD40XF |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50346788

(CHEMBL1795441)Show InChI InChI=1S/C19H18O3/c1-19(2,20)18-10-14-8-13-9-15(12-6-4-3-5-7-12)21-16(13)11-17(14)22-18/h3-9,11,18,20H,10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay |
Eur J Med Chem 46: 2386-96 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.022
BindingDB Entry DOI: 10.7270/Q2WD40XF |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50005781
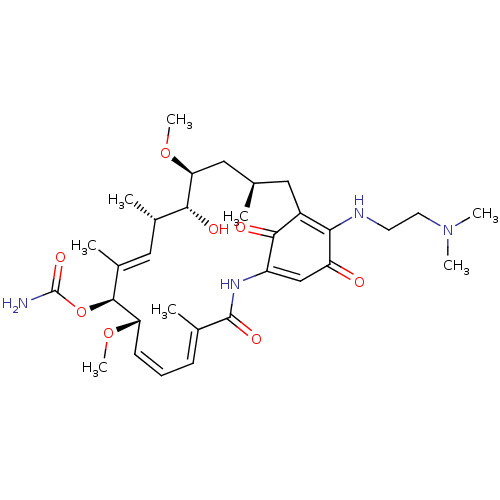
(ALVESPIMYCIN | CHEBI:65324)Show SMILES CO[C@H]1C[C@H](C)CC2=C(NCCN(C)C)C(=O)C=C(NC(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)C2=O |r,c:7,25,t:17,23,36| Show InChI InChI=1S/C32H48N4O8/c1-18-14-22-27(34-12-13-36(5)6)24(37)17-23(29(22)39)35-31(40)19(2)10-9-11-25(42-7)30(44-32(33)41)21(4)16-20(3)28(38)26(15-18)43-8/h9-11,16-18,20,25-26,28,30,34,38H,12-15H2,1-8H3,(H2,33,41)(H,35,40)/b11-9-,19-10+,21-16+/t18-,20+,25+,26+,28-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Research Institute of Biosciences and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha protein accumulation in human Hep3B cells treated for 30 mins measured after 12 hrs by Western blot analysis |
J Nat Prod 73: 1167-9 (2010)
Article DOI: 10.1021/np900820p
BindingDB Entry DOI: 10.7270/Q2TH8QHP |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50005781

(ALVESPIMYCIN | CHEBI:65324)Show SMILES CO[C@H]1C[C@H](C)CC2=C(NCCN(C)C)C(=O)C=C(NC(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)C2=O |r,c:7,25,t:17,23,36| Show InChI InChI=1S/C32H48N4O8/c1-18-14-22-27(34-12-13-36(5)6)24(37)17-23(29(22)39)35-31(40)19(2)10-9-11-25(42-7)30(44-32(33)41)21(4)16-20(3)28(38)26(15-18)43-8/h9-11,16-18,20,25-26,28,30,34,38H,12-15H2,1-8H3,(H2,33,41)(H,35,40)/b11-9-,19-10+,21-16+/t18-,20+,25+,26+,28-,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Research Institute of Biosciences and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha protein accumulation in human Hep3B cells treated for 30 mins measured after 12 hrs by Western blot analysis |
J Nat Prod 72: 39-43 (2009)
Article DOI: 10.1021/np800491u
BindingDB Entry DOI: 10.7270/Q27947GK |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50008935
((20S)-10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4ccc(O)c(CN(C)C)c4cc3Cn1c2=O |r| Show InChI InChI=1S/C23H23N3O5/c1-4-23(30)16-8-18-20-12(9-26(18)21(28)15(16)11-31-22(23)29)7-13-14(10-25(2)3)19(27)6-5-17(13)24-20/h5-8,27,30H,4,9-11H2,1-3H3/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIF1alpha in human U251 cells under hypoxic condition by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 6426-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.083
BindingDB Entry DOI: 10.7270/Q2HX1CXM |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50576962

(CHEMBL4849133)Show SMILES [H][C@@]12C[C@@](C)(CC[C@]1(C)CC[C@]1(C)C3=CC=C4C(C)=C(O)C(=O)C=C4[C@]3(C)CC[C@@]21C)C(=O)OCC(=O)Nc1cc(C)on1 |r,c:24,t:14,16,19| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hypoxia-induced HIF1alpha transcriptional activity in human Hep3B cells co-transfected with luciferase reporter plasmid containing six ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113474
BindingDB Entry DOI: 10.7270/Q2WQ07MW |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50593335

(CHEMBL5192165) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00308
BindingDB Entry DOI: 10.7270/Q2G73JR0 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50576965

(CHEMBL4852306)Show SMILES [H][C@@]12C[C@@](C)(CC[C@]1(C)CC[C@]1(C)C3=CC=C4C(C)=C(O)C(=O)C=C4[C@]3(C)CC[C@@]21C)C(=O)OCCOc1ccc2ccc(=O)oc2c1 |r,c:24,t:14,16,19| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hypoxia-induced HIF1alpha transcriptional activity in human Hep3B cells co-transfected with luciferase reporter plasmid containing six ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113474
BindingDB Entry DOI: 10.7270/Q2WQ07MW |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50354121

(CHEMBL1836238)Show SMILES CCOc1ccccc1-n1c(CN2CCNCC2)nc2ccccc2c1=O |(5.33,.38,;4,-.38,;2.67,.38,;2.67,1.93,;4,2.7,;4,4.23,;2.67,5.01,;1.33,4.23,;1.33,2.7,;,1.93,;,.38,;1.33,-.39,;1.33,-1.93,;,-2.7,;,-4.23,;1.33,-5.01,;2.67,-4.24,;2.67,-2.7,;-1.33,-.39,;-2.67,.38,;-4,-.39,;-5.33,.38,;-5.33,1.93,;-4,2.7,;-2.67,1.93,;-1.33,2.7,;-1.33,4.23,)| Show InChI InChI=1S/C21H24N4O2/c1-2-27-19-10-6-5-9-18(19)25-20(15-24-13-11-22-12-14-24)23-17-8-4-3-7-16(17)21(25)26/h3-10,22H,2,11-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of hypoxia-induced HIF1alpha activation in human ME180 cells by HRE3-Bla-luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 5239-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.043
BindingDB Entry DOI: 10.7270/Q2H70G65 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data