 Found 9 hits Enz. Inhib. hit(s) with Target = 'Glycogen phosphorylase, liver form' AND taxid = 10090
Found 9 hits Enz. Inhib. hit(s) with Target = 'Glycogen phosphorylase, liver form' AND taxid = 10090 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50149207

(2N-(2-{2-[2-(5-chloro-1H-2-indolylcarboxamido)etho...)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)NCCOCCOCCNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C24H24Cl2N4O4/c25-17-1-3-19-15(11-17)13-21(29-19)23(31)27-5-7-33-9-10-34-8-6-28-24(32)22-14-16-12-18(26)2-4-20(16)30-22/h1-4,11-14,29-30H,5-10H2,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50135550
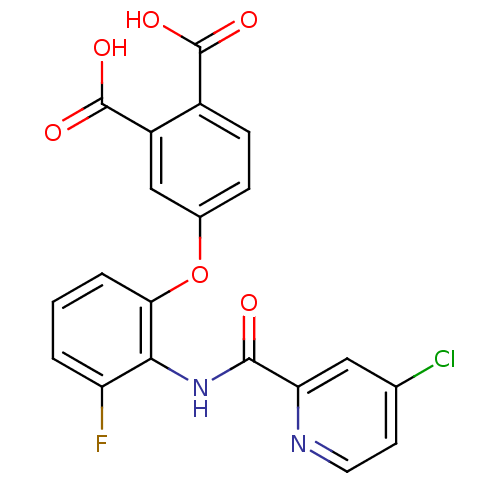
(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-3-fluo...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H12ClFN2O6/c21-10-6-7-23-15(8-10)18(25)24-17-14(22)2-1-3-16(17)30-11-4-5-12(19(26)27)13(9-11)20(28)29/h1-9H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50135552
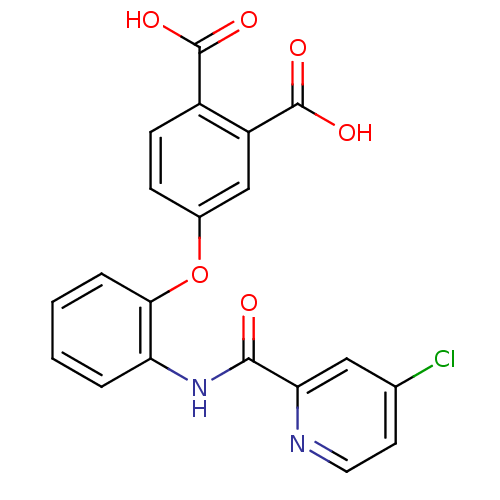
(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-phenox...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H13ClN2O6/c21-11-7-8-22-16(9-11)18(24)23-15-3-1-2-4-17(15)29-12-5-6-13(19(25)26)14(10-12)20(27)28/h1-10H,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50135563
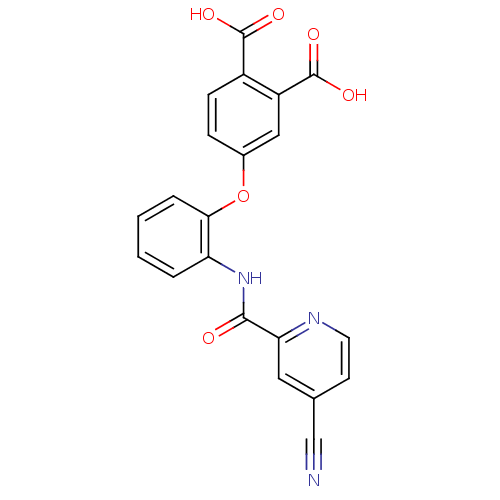
(4-{2-[(4-Cyano-pyridine-2-carbonyl)-amino]-phenoxy...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(ccn2)C#N)cc1C(O)=O Show InChI InChI=1S/C21H13N3O6/c22-11-12-7-8-23-17(9-12)19(25)24-16-3-1-2-4-18(16)30-13-5-6-14(20(26)27)15(10-13)21(28)29/h1-10H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50135556
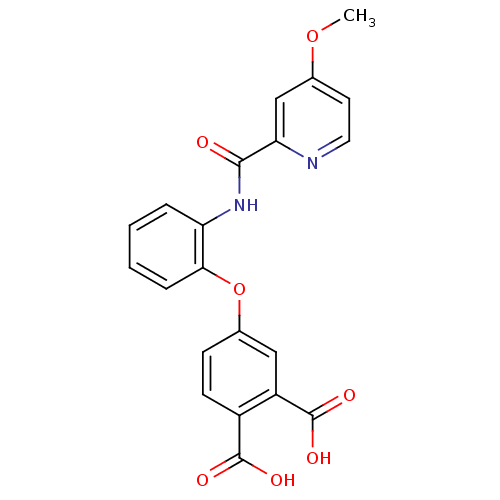
(4-{2-[(4-Methoxy-pyridine-2-carbonyl)-amino]-pheno...)Show SMILES COc1ccnc(c1)C(=O)Nc1ccccc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C21H16N2O7/c1-29-12-8-9-22-17(11-12)19(24)23-16-4-2-3-5-18(16)30-13-6-7-14(20(25)26)15(10-13)21(27)28/h2-11H,1H3,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50065965
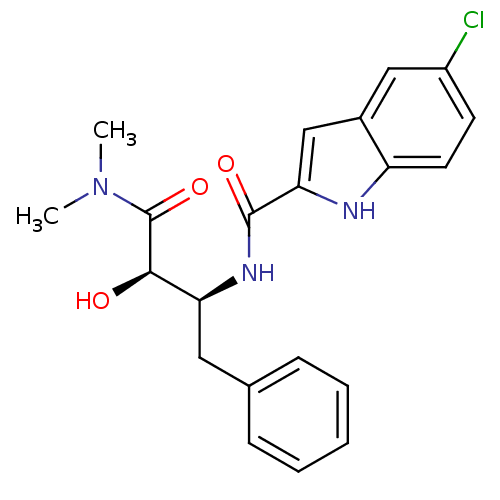
(5-Chloro-1H-indole-2-carboxylic acid ((1S,2R)-1-be...)Show SMILES CN(C)C(=O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C21H22ClN3O3/c1-25(2)21(28)19(26)17(10-13-6-4-3-5-7-13)24-20(27)18-12-14-11-15(22)8-9-16(14)23-18/h3-9,11-12,17,19,23,26H,10H2,1-2H3,(H,24,27)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50135549
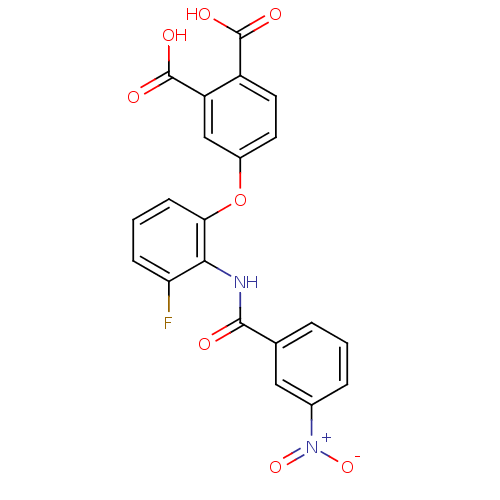
(4-[3-Fluoro-2-(3-nitro-benzoylamino)-phenoxy]-phth...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cccc(c2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C21H13FN2O8/c22-16-5-2-6-17(32-13-7-8-14(20(26)27)15(10-13)21(28)29)18(16)23-19(25)11-3-1-4-12(9-11)24(30)31/h1-10H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50149237
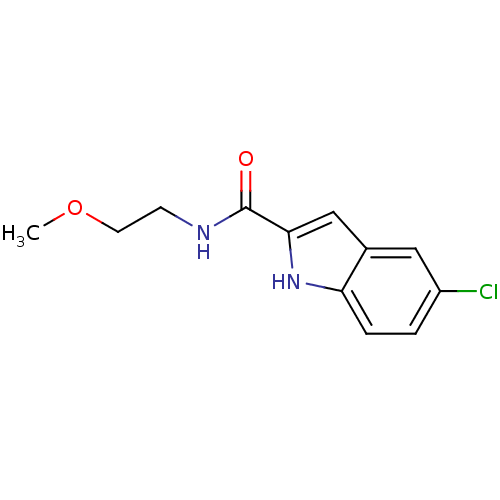
(5-Chloro-1H-indole-2-carboxylic acid (2-methoxy-et...)Show InChI InChI=1S/C12H13ClN2O2/c1-17-5-4-14-12(16)11-7-8-6-9(13)2-3-10(8)15-11/h2-3,6-7,15H,4-5H2,1H3,(H,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50560998
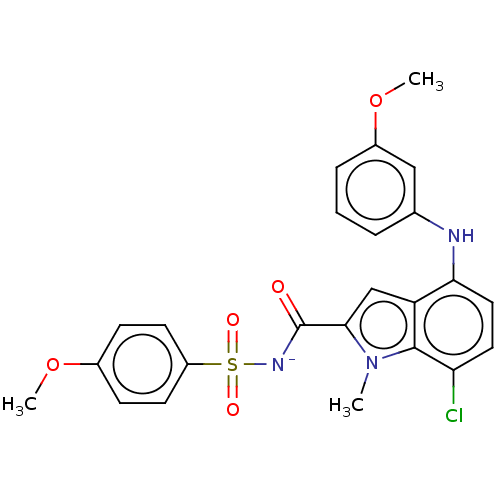
(CHEMBL4759496)Show SMILES [Na;v0+].[#6]-[#8]-c1ccc(cc1)S(=O)(=O)[#7-]-[#6](=O)-c1cc2c(-[#7]-c3cccc(-[#8]-[#6])c3)ccc(Cl)c2n1-[#6] | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of mouse liver Glycogen phosphorylase |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00726
BindingDB Entry DOI: 10.7270/Q2DR306R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data