 Found 1140 hits Enz. Inhib. hit(s) with Target = 'Mu-type opioid receptor' AND taxid = 10090
Found 1140 hits Enz. Inhib. hit(s) with Target = 'Mu-type opioid receptor' AND taxid = 10090 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(MOUSE) | CHEMBL5287792

Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CN(C)C)o1 Show InChI InChI=1S/C26H38N6O4/c1-30(2)17-18-11-12-21(36-18)25(33)31(3)13-9-7-8-10-14-32(4)26-28-20-16-23(35-6)22(34-5)15-19(20)24(27)29-26/h11-12,15-16H,7-10,13-14,17H2,1-6H3,(H2,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | CHEMBL5277326

Show InChI InChI=1S/C20H24N2OS/c1-4-18(23)15-10-11-20-17(14-15)22(13-7-12-21(2)3)16-8-5-6-9-19(16)24-20/h5-6,8-11,14H,4,7,12-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor in rat aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033531
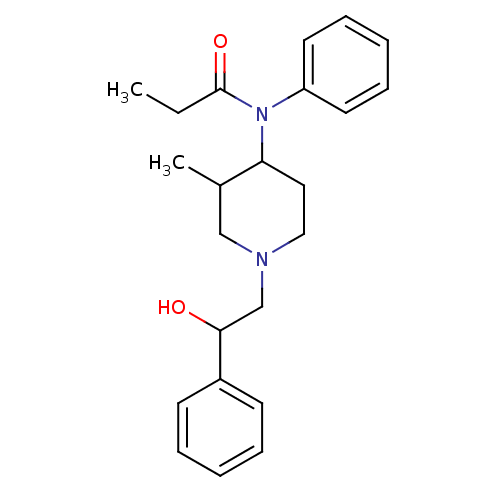
(CHEMBL333410 | N-[1-(2-Hydroxy-2-phenyl-ethyl)-3-m...)Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM21865
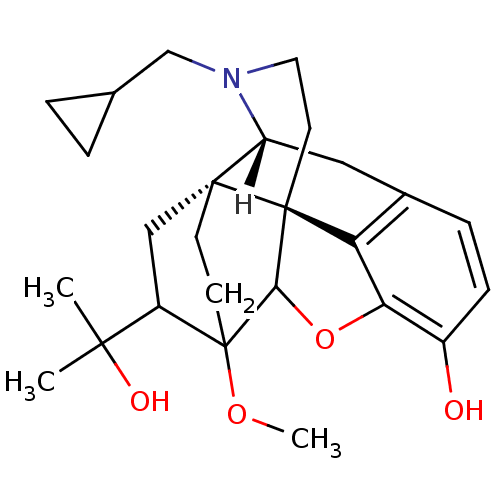
((14beta)-17-(cyclopropylmethyl)-18-(1-hydroxy-1-me...)Show SMILES [H][C@]12Cc3ccc(O)c4OC5[C@](CCN1CC1CC1)(c34)[C@@]21CCC5(OC)C(C1)C(C)(C)O |TLB:28:26:11.10:21.22,8:19:20:14.12.13,4:3:20:14.12.13| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18?,19-,22?,24-,25+,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50380909
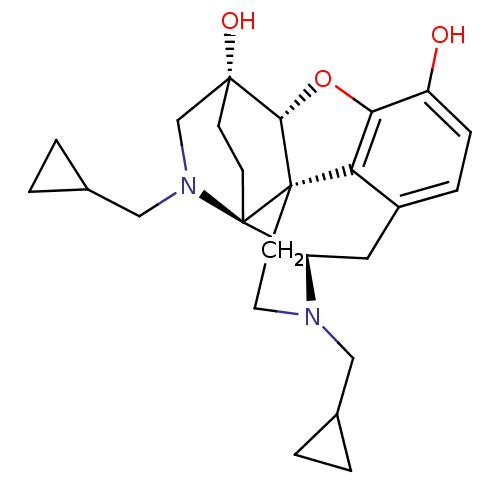
(CHEMBL2016678)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@]1(O)CC[C@@]35N(CC2CC2)C1 |r,THB:25:24:22.21:14.15| Show InChI InChI=1S/C25H32N2O3/c28-18-6-5-17-11-19-25-8-7-23(29,14-27(25)13-16-3-4-16)22-24(25,20(17)21(18)30-22)9-10-26(19)12-15-1-2-15/h5-6,15-16,19,22,28-29H,1-4,7-14H2/t19-,22+,23-,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mouse brain mu opioid receptor |
Bioorg Med Chem Lett 22: 2689-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.001
BindingDB Entry DOI: 10.7270/Q24X58T4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50102826
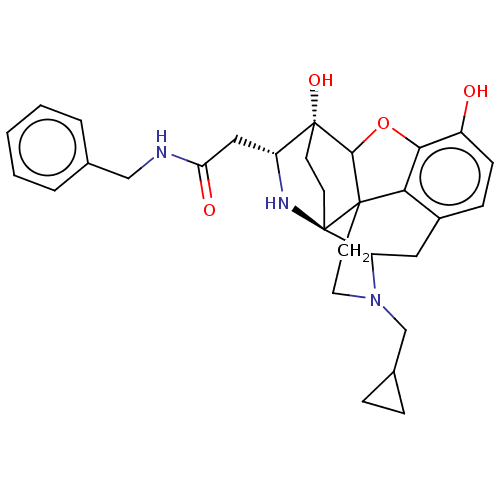
(CHEMBL3339374)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29-,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491882
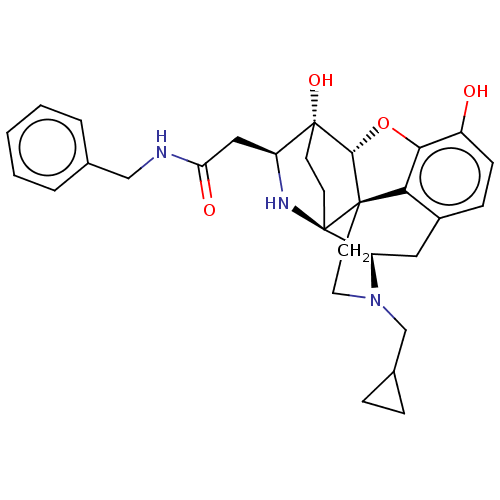
(CHEMBL3215908)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23+,27+,28+,29+,30+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033533

(CHEMBL121403 | N-[(3R,4R)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86416

(Dmt-d-Arg-Phe-A2pr-NH2)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C29H43N9O5/c1-16-11-19(39)12-17(2)20(16)14-21(31)26(41)36-22(9-6-10-35-29(33)34)27(42)37-23(13-18-7-4-3-5-8-18)28(43)38-24(15-30)25(32)40/h3-5,7-8,11-12,21-24,39H,6,9-10,13-15,30-31H2,1-2H3,(H2,32,40)(H,36,41)(H,37,42)(H,38,43)(H4,33,34,35)/t21-,22+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0636 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491892

(CHEMBL3216338)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(O)[C@@H](N1)C(=O)Nc1ccccc1)ccc3O |r,THB:26:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C28H31N3O4.2ClH/c32-19-9-8-17-14-20-28-11-10-27(34,23(30-28)24(33)29-18-4-2-1-3-5-18)25-26(28,21(17)22(19)35-25)12-13-31(20)15-16-6-7-16;;/h1-5,8-9,16,20,23,25,30,32,34H,6-7,10-15H2,(H,29,33);2*1H/t20-,23+,25-,26+,27-,28-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033534
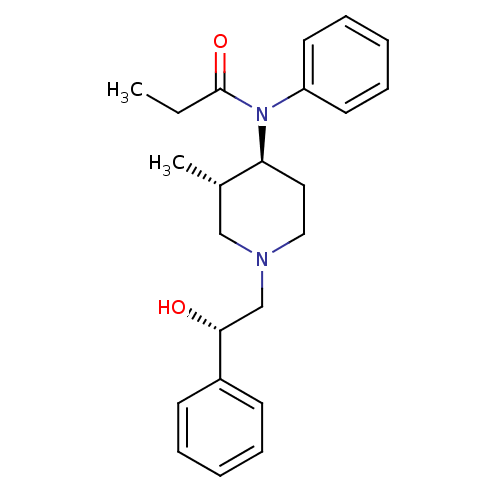
(CHEMBL338510 | N-[(3S,4S)-1-((S)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491883

(CHEMBL3216579)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)Nc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C29H33N3O4.2ClH/c33-20-9-8-18-14-22-29-11-10-28(35,21(31-29)15-23(34)30-19-4-2-1-3-5-19)26-27(29,24(18)25(20)36-26)12-13-32(22)16-17-6-7-17;;/h1-5,8-9,17,21-22,26,31,33,35H,6-7,10-16H2,(H,30,34);2*1H/t21-,22+,26+,27+,28+,29+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50380906
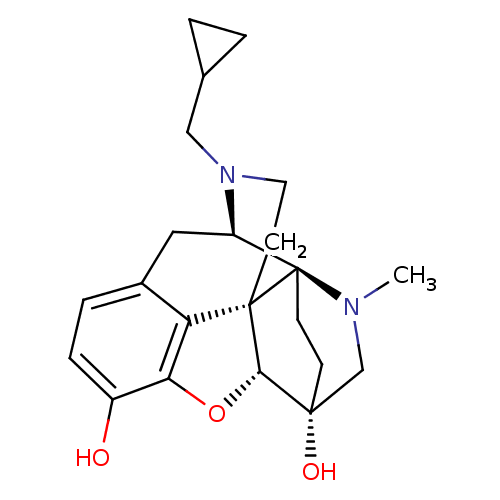
(CHEMBL2016675)Show SMILES CN1C[C@]2(O)CC[C@]11[C@H]3Cc4ccc(O)c5O[C@@H]2[C@]1(CCN3CC1CC1)c45 |r,TLB:0:1:6.5:18.17| Show InChI InChI=1S/C22H28N2O3/c1-23-12-20(26)6-7-22(23)16-10-14-4-5-15(25)18-17(14)21(22,19(20)27-18)8-9-24(16)11-13-2-3-13/h4-5,13,16,19,25-26H,2-3,6-12H2,1H3/t16-,19+,20-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mouse brain mu opioid receptor |
Bioorg Med Chem Lett 22: 2689-92 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.001
BindingDB Entry DOI: 10.7270/Q24X58T4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50493749

(CHEMBL2437063)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]5([H])C=C[C@H]2NC(=O)\C=C\c1ccccc1)ccc3O |r,c:19,THB:10:9:6.4.5:14| Show InChI InChI=1S/C26H26N2O3/c1-28-14-13-26-18-9-10-19(27-22(30)12-7-16-5-3-2-4-6-16)25(26)31-24-21(29)11-8-17(23(24)26)15-20(18)28/h2-12,18-20,25,29H,13-15H2,1H3,(H,27,30)/b12-7+/t18-,19+,20+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned mu opioid receptor expressed in CHO cells after 90 mins |
Eur J Med Chem 69: 786-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.031
BindingDB Entry DOI: 10.7270/Q2MK6GTW |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50177898

(2-Fluoro-N-[4-methoxymethyl-1-(2-thiophen-2-yl-eth...)Show SMILES COCC1(CCN(CCc2cccs2)CC1)N(C(=O)C(C)F)c1ccccc1 Show InChI InChI=1S/C22H29FN2O2S/c1-18(23)21(26)25(19-7-4-3-5-8-19)22(17-27-2)11-14-24(15-12-22)13-10-20-9-6-16-28-20/h3-9,16,18H,10-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen
Curated by ChEMBL
| Assay Description
Binding constant for Opioid receptor mu 1 in mouse |
Bioorg Med Chem Lett 15: 1773-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.049
BindingDB Entry DOI: 10.7270/Q28916M4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50395117
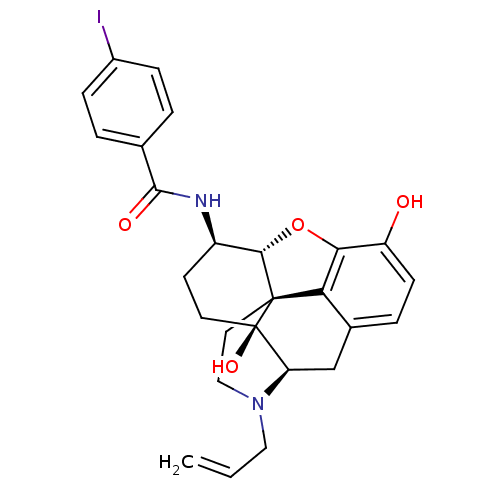
(CHEMBL2163536)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(I)cc1 |r| Show InChI InChI=1S/C26H27IN2O4/c1-2-12-29-13-11-25-21-16-5-8-19(30)22(21)33-23(25)18(9-10-26(25,32)20(29)14-16)28-24(31)15-3-6-17(27)7-4-15/h2-8,18,20,23,30,32H,1,9-14H2,(H,28,31)/t18-,20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned MOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491897

(CHEMBL2387739)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)Nc2ccccc2)N1C(C)=O)ccc3O |r,THB:34:18:5.7.6:16.10.15,35:34:2.17:19.20,4:5:18:16.10.15,24:23:2.17:19.20| Show InChI InChI=1S/C31H35N3O5.ClH/c1-18(35)34-23(16-25(37)32-21-5-3-2-4-6-21)30(38)11-12-31(34)24-15-20-9-10-22(36)27-26(20)29(31,28(30)39-27)13-14-33(24)17-19-7-8-19;/h2-6,9-10,19,23-24,28,36,38H,7-8,11-17H2,1H3,(H,32,37);1H/t23-,24+,28+,29+,30+,31+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50346951
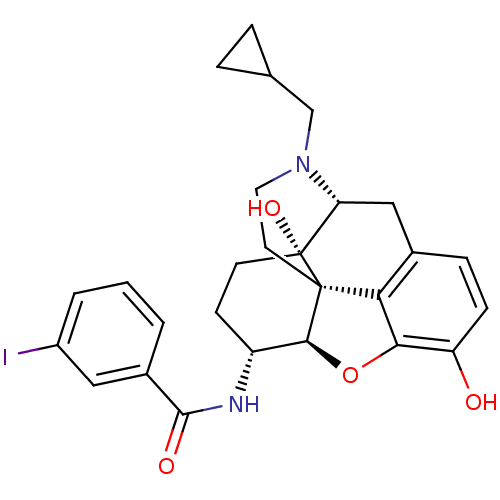
(CHEMBL1795711 | CHEMBL1795714)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1cccc(I)c1 |r| Show InChI InChI=1S/C27H29IN2O4/c28-18-3-1-2-17(12-18)25(32)29-19-8-9-27(33)21-13-16-6-7-20(31)23-22(16)26(27,24(19)34-23)10-11-30(21)14-15-4-5-15/h1-3,6-7,12,15,19,21,24,31,33H,4-5,8-11,13-14H2,(H,29,32)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned MOR-1 expressed in CHO cell membrane after 90 mins |
J Med Chem 55: 6352-62 (2012)
Article DOI: 10.1021/jm300305c
BindingDB Entry DOI: 10.7270/Q2930V8C |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50493758
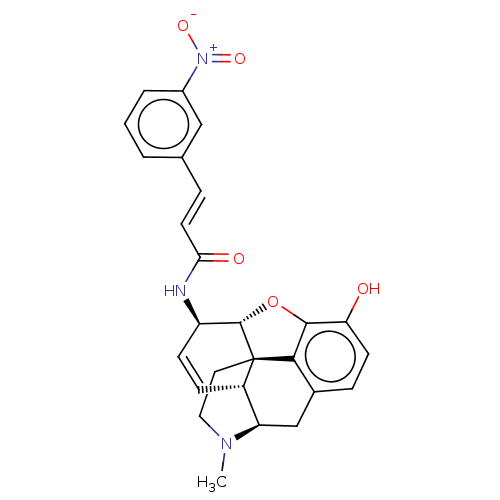
(CHEMBL2437067)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]5([H])C=C[C@H]2NC(=O)\C=C\c1cccc(c1)[N+]([O-])=O)ccc3O |r,c:19,THB:10:9:6.4.5:14| Show InChI InChI=1S/C26H25N3O5/c1-28-12-11-26-18-7-8-19(27-22(31)10-5-15-3-2-4-17(13-15)29(32)33)25(26)34-24-21(30)9-6-16(23(24)26)14-20(18)28/h2-10,13,18-20,25,30H,11-12,14H2,1H3,(H,27,31)/b10-5+/t18-,19+,20+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned mu opioid receptor expressed in CHO cells after 90 mins |
Eur J Med Chem 69: 786-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.031
BindingDB Entry DOI: 10.7270/Q2MK6GTW |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50189257
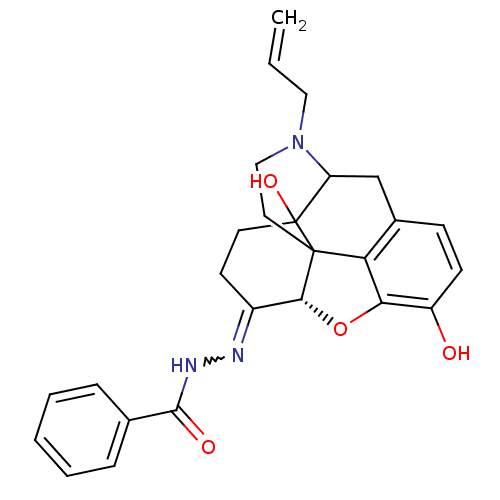
(CHEMBL378753 | NalBzOH)Show SMILES Oc1ccc2CC3N(CC=C)CCC45[C@H](Oc1c24)C(CCC35O)=NNC(=O)c1ccccc1 |w:23.28,TLB:22:21:7.11.12:17.5.4,THB:8:7:21:17.5.4,16:17:21:7.11.12| Show InChI InChI=1S/C26H27N3O4/c1-2-13-29-14-12-25-21-17-8-9-19(30)22(21)33-23(25)18(10-11-26(25,32)20(29)15-17)27-28-24(31)16-6-4-3-5-7-16/h2-9,20,23,30,32H,1,10-15H2,(H,28,31)/t20?,23-,25?,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM21865

((14beta)-17-(cyclopropylmethyl)-18-(1-hydroxy-1-me...)Show SMILES [H][C@]12Cc3ccc(O)c4OC5[C@](CCN1CC1CC1)(c34)[C@@]21CCC5(OC)C(C1)C(C)(C)O |TLB:28:26:11.10:21.22,8:19:20:14.12.13,4:3:20:14.12.13| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18?,19-,22?,24-,25+,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491885
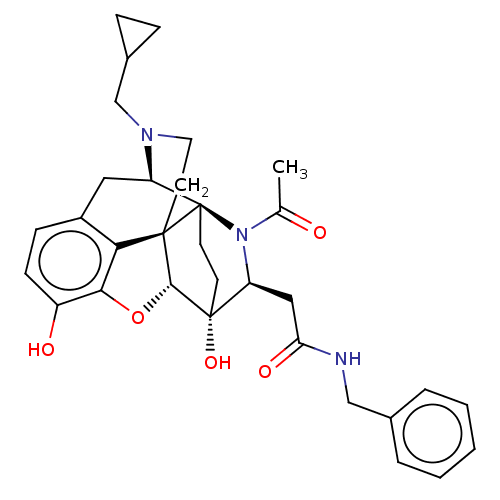
(CHEMBL2387740)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@H](CC(=O)NCc2ccccc2)N1C(C)=O)ccc3O |r,THB:35:18:5.7.6:16.10.15,4:5:18:16.10.15,24:23:2.17:19.20,36:35:2.17:19.20| Show InChI InChI=1S/C32H37N3O5.ClH/c1-19(36)35-24(16-26(38)33-17-20-5-3-2-4-6-20)31(39)11-12-32(35)25-15-22-9-10-23(37)28-27(22)30(32,29(31)40-28)13-14-34(25)18-21-7-8-21;/h2-6,9-10,21,24-25,29,37,39H,7-8,11-18H2,1H3,(H,33,38);1H/t24-,25+,29+,30+,31+,32+;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50506207

(CHEMBL4466064)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)c1coc2ccccc12)ccc3O |r,THB:11:10:18:7.6.5| Show InChI InChI=1S/C29H30N2O5.ClH/c32-21-8-7-17-13-23-29(34)10-9-20(30-27(33)19-15-35-22-4-2-1-3-18(19)22)26-28(29,24(17)25(21)36-26)11-12-31(23)14-16-5-6-16;/h1-4,7-8,15-16,20,23,26,32,34H,5-6,9-14H2,(H,30,33);1H/t20-,23-,26+,28+,29-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay |
J Med Chem 62: 11399-11415 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01767
BindingDB Entry DOI: 10.7270/Q2G73J1K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50102828
(CHEMBL3339372)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@H]1CC(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,17:18:23:7.12.13,22:23:4.18.5:7.12.13,24:23:4.18.5:7.12.13,8:7:4.18.5:23| Show InChI InChI=1S/C30H35N3O4/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35)/t22-,23?,27?,28?,29+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033536

(CHEMBL121494 | N-[(3R,4R)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@@H]1CCN(C[C@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50491884

(CHEMBL3216801)Show SMILES Cl.Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14[C@@]51CC[C@@]2(O)[C@@H](CC(=O)NCc2ccccc2)N1)ccc3O |r,THB:25:24:3.18:20.21,12:11:19:6.8.7| Show InChI InChI=1S/C30H35N3O4.2ClH/c34-21-9-8-20-14-23-30-11-10-29(36,22(32-30)15-24(35)31-16-18-4-2-1-3-5-18)27-28(30,25(20)26(21)37-27)12-13-33(23)17-19-6-7-19;;/h1-5,8-9,19,22-23,27,32,34,36H,6-7,10-17H2,(H,31,35);2*1H/t22-,23-,27-,28-,29-,30-;;/m1../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse whole brain devoid of cerebellum |
Bioorg Med Chem 21: 3032-50 (2013)
Article DOI: 10.1016/j.bmc.2013.03.026
BindingDB Entry DOI: 10.7270/Q2JQ13XJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50506210
(CHEMBL4455793)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)c1csc2ccccc12)ccc3O |r,THB:11:10:18:7.6.5| Show InChI InChI=1S/C29H30N2O4S.ClH/c32-21-8-7-17-13-23-29(34)10-9-20(30-27(33)19-15-36-22-4-2-1-3-18(19)22)26-28(29,24(17)25(21)35-26)11-12-31(23)14-16-5-6-16;/h1-4,7-8,15-16,20,23,26,32,34H,5-6,9-14H2,(H,30,33);1H/t20-,23-,26+,28+,29-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay |
J Med Chem 62: 11399-11415 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01767
BindingDB Entry DOI: 10.7270/Q2G73J1K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50506217
(CHEMBL4458688)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)c1cc2ccccc2s1)ccc3O |r,THB:11:10:18:7.6.5| Show InChI InChI=1S/C29H30N2O4S.ClH/c32-20-8-7-18-14-23-29(34)10-9-19(30-27(33)22-13-17-3-1-2-4-21(17)36-22)26-28(29,24(18)25(20)35-26)11-12-31(23)15-16-5-6-16;/h1-4,7-8,13,16,19,23,26,32,34H,5-6,9-12,14-15H2,(H,30,33);1H/t19-,23-,26+,28+,29-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay |
J Med Chem 62: 11399-11415 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01767
BindingDB Entry DOI: 10.7270/Q2G73J1K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50102833
(CHEMBL3339378)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N=C1C(=O)NCc1ccccc1 |r,c:30,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,8:7:4.18.5:23,17:18:23:7.12.13,24:23:4.18.5:7.12.13,22:23:4.18.5:7.12.13| Show InChI InChI=1S/C29H31N3O4/c33-20-9-8-19-14-21-29-11-10-28(35,24(31-29)25(34)30-15-17-4-2-1-3-5-17)26-27(29,22(19)23(20)36-26)12-13-32(21)16-18-6-7-18/h1-5,8-9,18,21,26,33,35H,6-7,10-16H2,(H,30,34)/t21?,26?,27?,28-,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50506225

(CHEMBL4562210)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)c1ccc2sccc2c1)ccc3O |r,THB:11:10:18:7.6.5| Show InChI InChI=1S/C29H30N2O4S.ClH/c32-21-5-3-18-14-23-29(34)9-7-20(30-27(33)19-4-6-22-17(13-19)8-12-36-22)26-28(29,24(18)25(21)35-26)10-11-31(23)15-16-1-2-16;/h3-6,8,12-13,16,20,23,26,32,34H,1-2,7,9-11,14-15H2,(H,30,33);1H/t20-,23-,26+,28+,29-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay |
J Med Chem 62: 11399-11415 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01767
BindingDB Entry DOI: 10.7270/Q2G73J1K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM85731
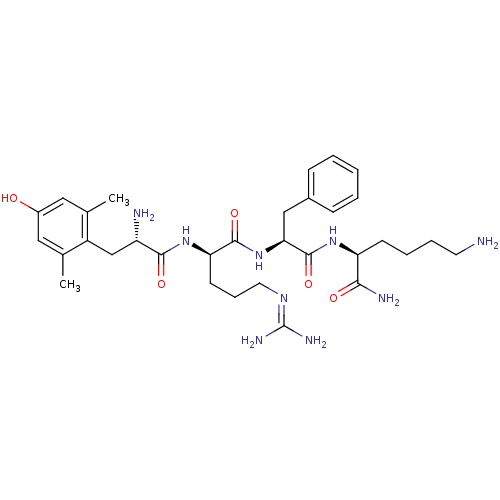
([Dmt1]DALDA)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86416

(Dmt-d-Arg-Phe-A2pr-NH2)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C29H43N9O5/c1-16-11-19(39)12-17(2)20(16)14-21(31)26(41)36-22(9-6-10-35-29(33)34)27(42)37-23(13-18-7-4-3-5-8-18)28(43)38-24(15-30)25(32)40/h3-5,7-8,11-12,21-24,39H,6,9-10,13-15,30-31H2,1-2H3,(H2,32,40)(H,36,41)(H,37,42)(H,38,43)(H4,33,34,35)/t21-,22+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50506211
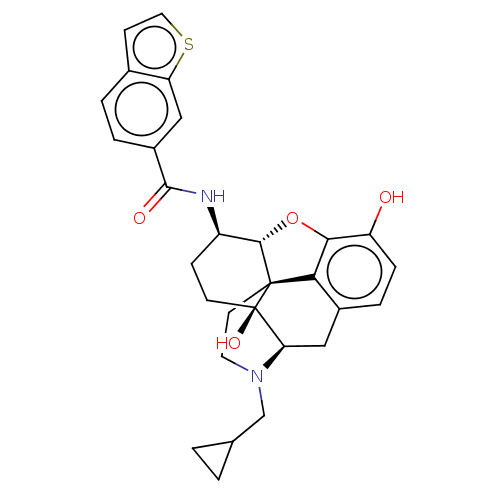
(CHEMBL4444746)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)c1ccc2ccsc2c1)ccc3O |r,THB:11:10:18:7.6.5| Show InChI InChI=1S/C29H30N2O4S.ClH/c32-21-6-5-18-14-23-29(34)9-7-20(30-27(33)19-4-3-17-8-12-36-22(17)13-19)26-28(29,24(18)25(21)35-26)10-11-31(23)15-16-1-2-16;/h3-6,8,12-13,16,20,23,26,32,34H,1-2,7,9-11,14-15H2,(H,30,33);1H/t20-,23-,26+,28+,29-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay |
J Med Chem 62: 11399-11415 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01767
BindingDB Entry DOI: 10.7270/Q2G73J1K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50506219
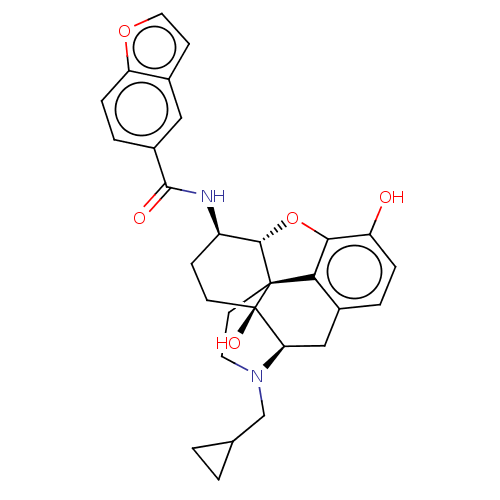
(CHEMBL4460495)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)c1ccc2occc2c1)ccc3O |r,THB:11:10:18:7.6.5| Show InChI InChI=1S/C29H30N2O5.ClH/c32-21-5-3-18-14-23-29(34)9-7-20(30-27(33)19-4-6-22-17(13-19)8-12-35-22)26-28(29,24(18)25(21)36-26)10-11-31(23)15-16-1-2-16;/h3-6,8,12-13,16,20,23,26,32,34H,1-2,7,9-11,14-15H2,(H,30,33);1H/t20-,23-,26+,28+,29-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay |
J Med Chem 62: 11399-11415 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01767
BindingDB Entry DOI: 10.7270/Q2G73J1K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50493755

(CHEMBL2437064)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@@]14[C@@]5([H])C=C[C@H]2NC(=O)\C=C\c1ccc(Cl)cc1)ccc3O |r,c:19,THB:10:9:6.4.5:14| Show InChI InChI=1S/C26H25ClN2O3/c1-29-13-12-26-18-8-9-19(28-22(31)11-4-15-2-6-17(27)7-3-15)25(26)32-24-21(30)10-5-16(23(24)26)14-20(18)29/h2-11,18-20,25,30H,12-14H2,1H3,(H,28,31)/b11-4+/t18-,19+,20+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BNtxA from mouse cloned mu opioid receptor expressed in CHO cells after 90 mins |
Eur J Med Chem 69: 786-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.031
BindingDB Entry DOI: 10.7270/Q2MK6GTW |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50102830
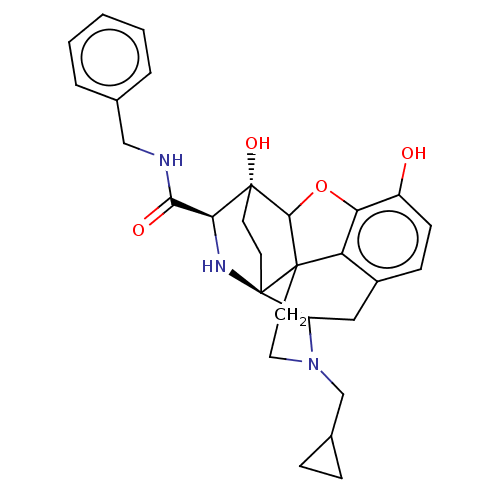
(CHEMBL3339381)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)[C@@]1(O)CC[C@@]35N[C@H]1C(=O)NCc1ccccc1 |r,TLB:26:25:14.15:22.21,THB:3:4:23:7.12.13,8:7:4.18.5:23,17:18:23:7.12.13,24:23:4.18.5:7.12.13,22:23:4.18.5:7.12.13| Show InChI InChI=1S/C29H33N3O4/c33-20-9-8-19-14-21-29-11-10-28(35,24(31-29)25(34)30-15-17-4-2-1-3-5-17)26-27(29,22(19)23(20)36-26)12-13-32(21)16-18-6-7-18/h1-5,8-9,18,21,24,26,31,33,35H,6-7,10-16H2,(H,30,34)/t21?,24-,26?,27?,28+,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in mouse brain membranes without cerebellum |
Bioorg Med Chem Lett 24: 4980-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.029
BindingDB Entry DOI: 10.7270/Q2154JTJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033530
(CHEMBL121211 | N-[(3S,4S)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50033530

(CHEMBL121211 | N-[(3S,4S)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@H](O)c2ccccc2)C[C@@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. |
J Med Chem 38: 3652-9 (1995)
BindingDB Entry DOI: 10.7270/Q2BP01V7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM85736
(Dmt-d-Arg-Phe-Orn-NH2 | H-Dmt-D-Arg-Phe-Orn-NH2)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C31H47N9O5/c1-18-14-21(41)15-19(2)22(18)17-23(33)28(43)39-25(11-7-13-37-31(35)36)29(44)40-26(16-20-8-4-3-5-9-20)30(45)38-24(27(34)42)10-6-12-32/h3-5,8-9,14-15,23-26,41H,6-7,10-13,16-17,32-33H2,1-2H3,(H2,34,42)(H,38,45)(H,39,43)(H,40,44)(H4,35,36,37)/t23-,24-,25+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50586108
(CHEMBL5094608)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@H]5N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)Cc1ccc[nH]1)ccc3O |r,THB:10:9:6.5.7:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-naloxone from mouse mu opioid receptor expressed in CHO cell membranes assessed as inhibition constant incubated for 1.5 hrs by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00087
BindingDB Entry DOI: 10.7270/Q21Z489F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50506228
(CHEMBL4570108)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)c1cc2ccccc2o1)ccc3O |r,THB:11:10:18:7.6.5| Show InChI InChI=1S/C29H30N2O5.ClH/c32-20-8-7-18-14-23-29(34)10-9-19(30-27(33)22-13-17-3-1-2-4-21(17)35-22)26-28(29,24(18)25(20)36-26)11-12-31(23)15-16-5-6-16;/h1-4,7-8,13,16,19,23,26,32,34H,5-6,9-12,14-15H2,(H,30,33);1H/t19-,23-,26+,28+,29-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay |
J Med Chem 62: 11399-11415 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01767
BindingDB Entry DOI: 10.7270/Q2G73J1K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50506231
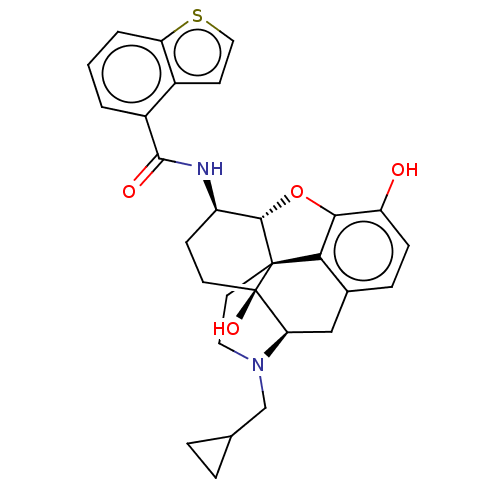
(CHEMBL4575933)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)c1cccc2sccc12)ccc3O |r,THB:11:10:18:7.6.5| Show InChI InChI=1S/C29H30N2O4S.ClH/c32-21-7-6-17-14-23-29(34)10-8-20(30-27(33)19-2-1-3-22-18(19)9-13-36-22)26-28(29,24(17)25(21)35-26)11-12-31(23)15-16-4-5-16;/h1-3,6-7,9,13,16,20,23,26,32,34H,4-5,8,10-12,14-15H2,(H,30,33);1H/t20-,23-,26+,28+,29-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay |
J Med Chem 62: 11399-11415 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01767
BindingDB Entry DOI: 10.7270/Q2G73J1K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM85736

(Dmt-d-Arg-Phe-Orn-NH2 | H-Dmt-D-Arg-Phe-Orn-NH2)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C31H47N9O5/c1-18-14-21(41)15-19(2)22(18)17-23(33)28(43)39-25(11-7-13-37-31(35)36)29(44)40-26(16-20-8-4-3-5-9-20)30(45)38-24(27(34)42)10-6-12-32/h3-5,8-9,14-15,23-26,41H,6-7,10-13,16-17,32-33H2,1-2H3,(H2,34,42)(H,38,45)(H,39,43)(H,40,44)(H4,35,36,37)/t23-,24-,25+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM85731

([Dmt1]DALDA)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50506218
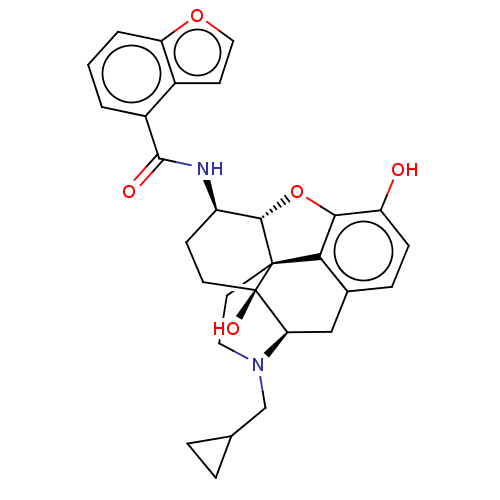
(CHEMBL4565323)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)c1cccc2occc12)ccc3O |r,THB:11:10:18:7.6.5| Show InChI InChI=1S/C29H30N2O5.ClH/c32-21-7-6-17-14-23-29(34)10-8-20(30-27(33)19-2-1-3-22-18(19)9-13-35-22)26-28(29,24(17)25(21)36-26)11-12-31(23)15-16-4-5-16;/h1-3,6-7,9,13,16,20,23,26,32,34H,4-5,8,10-12,14-15H2,(H,30,33);1H/t20-,23-,26+,28+,29-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay |
J Med Chem 62: 11399-11415 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01767
BindingDB Entry DOI: 10.7270/Q2G73J1K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50508336

(CHEMBL4530500)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)c1ccncc1[N+]([O-])=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C26H28N4O6/c31-19-4-3-15-11-20-26(33)7-5-17(28-24(32)16-6-9-27-12-18(16)30(34)35)23-25(26,21(15)22(19)36-23)8-10-29(20)13-14-1-2-14/h3-4,6,9,12,14,17,20,23,31,33H,1-2,5,7-8,10-11,13H2,(H,28,32)/t17-,20-,23+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-naloxone from mouse MOR expressed in CHO cell membranes by competitive radioligand binding assay |
J Med Chem 62: 561-574 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01158
BindingDB Entry DOI: 10.7270/Q2CN776T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50586084

(CHEMBL5094575)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)Cc1cn[nH]c1)ccc3O |r,THB:10:9:17:6.4.5| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to mouse mu opioid receptor expressed in CHO cells in presence of naltrexone incubated for 1.5 hrs by competitive radioligand bindin... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02185
BindingDB Entry DOI: 10.7270/Q25Q510Q |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86418
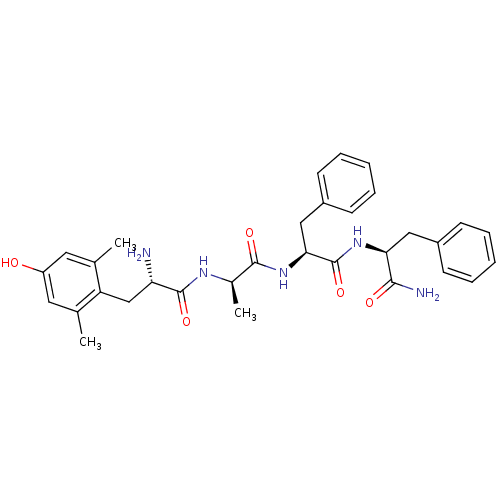
(Dmt-d-Ala-Phe-Phe-NH2)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C32H39N5O5/c1-19-14-24(38)15-20(2)25(19)18-26(33)31(41)35-21(3)30(40)37-28(17-23-12-8-5-9-13-23)32(42)36-27(29(34)39)16-22-10-6-4-7-11-22/h4-15,21,26-28,38H,16-18,33H2,1-3H3,(H2,34,39)(H,35,41)(H,36,42)(H,37,40)/t21-,26+,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86418

(Dmt-d-Ala-Phe-Phe-NH2)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C32H39N5O5/c1-19-14-24(38)15-20(2)25(19)18-26(33)31(41)35-21(3)30(40)37-28(17-23-12-8-5-9-13-23)32(42)36-27(29(34)39)16-22-10-6-4-7-11-22/h4-15,21,26-28,38H,16-18,33H2,1-3H3,(H2,34,39)(H,35,41)(H,36,42)(H,37,40)/t21-,26+,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50506213

(CHEMBL4457013)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC[C@H]2NC(=O)c1ccc2ccoc2c1)ccc3O |r,THB:11:10:18:7.6.5| Show InChI InChI=1S/C29H30N2O5.ClH/c32-21-6-5-18-14-23-29(34)9-7-20(30-27(33)19-4-3-17-8-12-35-22(17)13-19)26-28(29,24(18)25(21)36-26)10-11-31(23)15-16-1-2-16;/h3-6,8,12-13,16,20,23,26,32,34H,1-2,7,9-11,14-15H2,(H,30,33);1H/t20-,23-,26+,28+,29-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Naloxone from mouse mu opioid receptor expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay |
J Med Chem 62: 11399-11415 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01767
BindingDB Entry DOI: 10.7270/Q2G73J1K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data