

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus (strain A/Udorn/307/1972 H3N2)) | BDBM50401401 (CHEMBL2206459) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Research Center Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Udorn/1972(H3N2) neuraminidase in virus-infected allantoic fluid using 2'-(4-methylumbelliferyl)-alpha-D-N-acetylne... | J Med Chem 55: 8657-70 (2012) Article DOI: 10.1021/jm3008486 BindingDB Entry DOI: 10.7270/Q2B27WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Udorn/307/1972 H3N2)) | BDBM50401399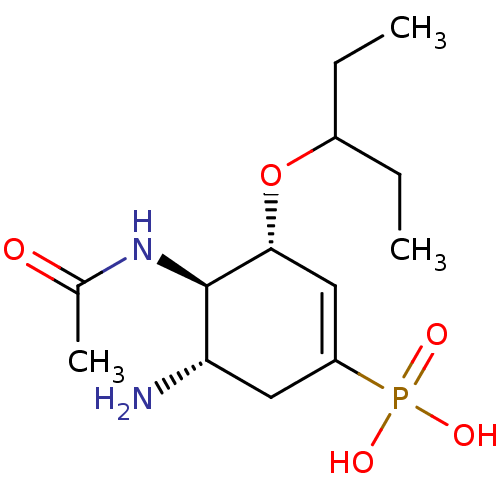 (CHEMBL2206456) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Research Center Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Udorn/1972(H3N2) neuraminidase in virus-infected allantoic fluid using 2'-(4-methylumbelliferyl)-alpha-D-N-acetylne... | J Med Chem 55: 8657-70 (2012) Article DOI: 10.1021/jm3008486 BindingDB Entry DOI: 10.7270/Q2B27WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Udorn/307/1972 H3N2)) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Research Center Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Udorn/1972(H3N2) neuraminidase in virus-infected allantoic fluid using 2'-(4-methylumbelliferyl)-alpha-D-N-acetylne... | J Med Chem 55: 8657-70 (2012) Article DOI: 10.1021/jm3008486 BindingDB Entry DOI: 10.7270/Q2B27WFN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (strain A/Udorn/307/1972 H3N2)) | BDBM50401398 (CHEMBL2206458) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Research Center Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Udorn/1972(H3N2) neuraminidase in virus-infected allantoic fluid using 2'-(4-methylumbelliferyl)-alpha-D-N-acetylne... | J Med Chem 55: 8657-70 (2012) Article DOI: 10.1021/jm3008486 BindingDB Entry DOI: 10.7270/Q2B27WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Udorn/307/1972 H3N2)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Research Center Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Udorn/1972(H3N2) neuraminidase in virus-infected allantoic fluid using 2'-(4-methylumbelliferyl)-alpha-D-N-acetylne... | J Med Chem 55: 8657-70 (2012) Article DOI: 10.1021/jm3008486 BindingDB Entry DOI: 10.7270/Q2B27WFN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (strain A/Udorn/307/1972 H3N2)) | BDBM50401400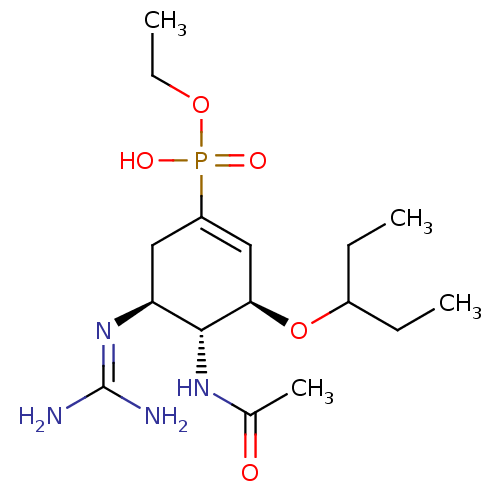 (CHEMBL2206932) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Research Center Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Udorn/1972(H3N2) neuraminidase in virus-infected allantoic fluid using 2'-(4-methylumbelliferyl)-alpha-D-N-acetylne... | J Med Chem 55: 8657-70 (2012) Article DOI: 10.1021/jm3008486 BindingDB Entry DOI: 10.7270/Q2B27WFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Udorn/307/1972 H3N2)) | BDBM50078329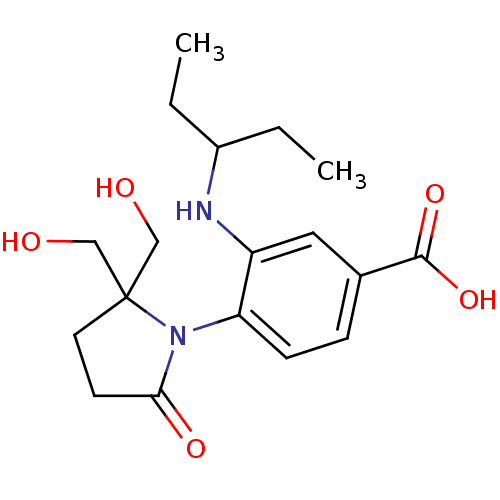 (1-[4-CARBOXY-2-(3-PENTYLAMINO)PHENYL]-5,5'-DI(HYDR...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/udorn/1972(H3N2)) neuraminidase using fluorogenic substrate 2'-(4-methylumbelliferyl)-alpha-D-N-acetylneuraminic a... | Bioorg Med Chem 20: 4582-9 (2012) Article DOI: 10.1016/j.bmc.2012.05.001 BindingDB Entry DOI: 10.7270/Q28S4QZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||