

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343888 ((R)-4-(4-(2,2-difluoro-1-hydroxyethyl)phenyl)-7-(4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of mouse P2Y14 receptor | Eur J Med Chem 175: 34-39 (2019) Article DOI: 10.1016/j.ejmech.2019.04.068 BindingDB Entry DOI: 10.7270/Q29C71R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of mouse P2Y14 receptor in presence of 2% HSA | Eur J Med Chem 175: 34-39 (2019) Article DOI: 10.1016/j.ejmech.2019.04.068 BindingDB Entry DOI: 10.7270/Q29C71R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343888 ((R)-4-(4-(2,2-difluoro-1-hydroxyethyl)phenyl)-7-(4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of mouse P2Y14 receptor in presence of 2% HSA | Eur J Med Chem 175: 34-39 (2019) Article DOI: 10.1016/j.ejmech.2019.04.068 BindingDB Entry DOI: 10.7270/Q29C71R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343131 (2-(2-cyanopyrimidin-5-yl)-N-(3-ethylphenyl)-4-o-to...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343129 (2-(4-cyanopyridin-3-yl)-N-(3-ethylphenyl)-4-o-toly...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343132 (2-(3,5-dimethylisoxazol-4-yl)-N-(3-ethylphenyl)-4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343886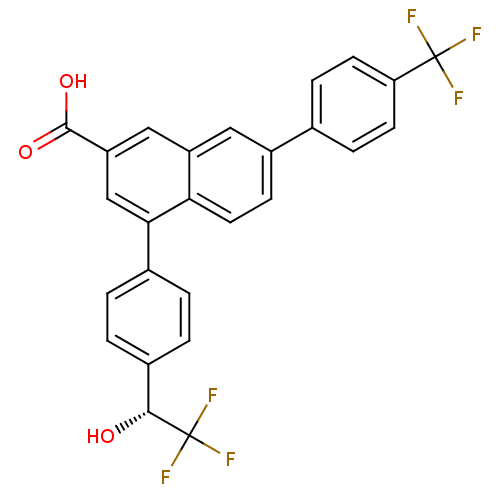 ((R)-4-(4-(2,2,2-trifluoro-1-hydroxyethyl)phenyl)-7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343888 ((R)-4-(4-(2,2-difluoro-1-hydroxyethyl)phenyl)-7-(4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343120 (4-(2,6-dimethylphenyl)-N-(3-ethylphenyl)-2-(pyridi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343128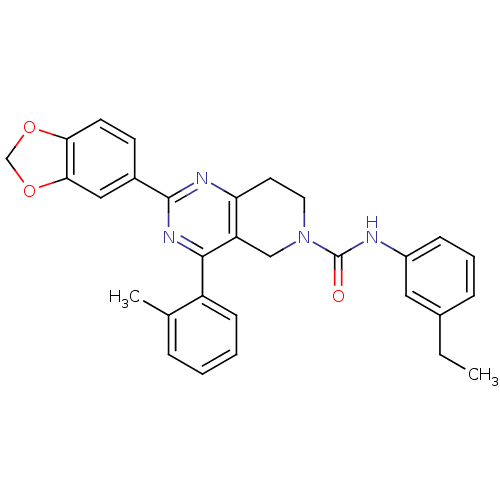 (2-(benzo[d][1,3]dioxol-5-yl)-N-(3-ethylphenyl)-4-o...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343130 (CHEMBL1771458 | N-(3-ethylphenyl)-2-(pyrimidin-5-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343127 (CHEMBL1771455 | N-(3-ethylphenyl)-2-(quinolin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50584883 (CHEMBL5074765) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to mouse P2Y14R expressed in HEK293 cells preincubated for 30 mins followed by addition of 6-amino-9-(2-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343885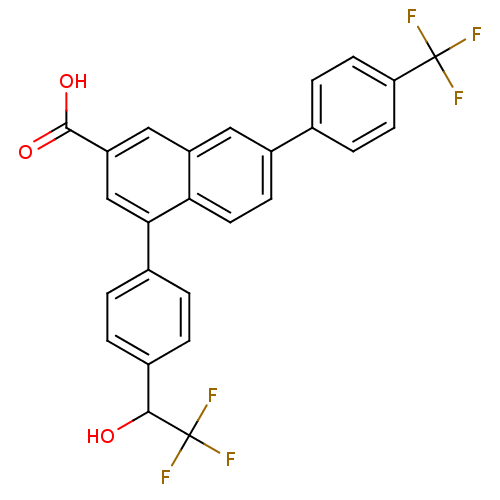 (4-(4-(2,2,2-trifluoro-1-hydroxyethyl)phenyl)-7-(4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343877 (7-(4-fluoro-2,6-dimethylbenzyloxy)-4-(thiophen-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of mouse P2Y14 receptor | Eur J Med Chem 175: 34-39 (2019) Article DOI: 10.1016/j.ejmech.2019.04.068 BindingDB Entry DOI: 10.7270/Q29C71R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343877 (7-(4-fluoro-2,6-dimethylbenzyloxy)-4-(thiophen-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343094 (2-(4-cyanophenyl)-N-(3-ethylphenyl)-4-o-tolyl-7,8-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50584890 (CHEMBL5079102) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to mouse P2Y14R expressed in HEK293 cells preincubated for 30 mins followed by addition of 6-amino-9-(2-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50584893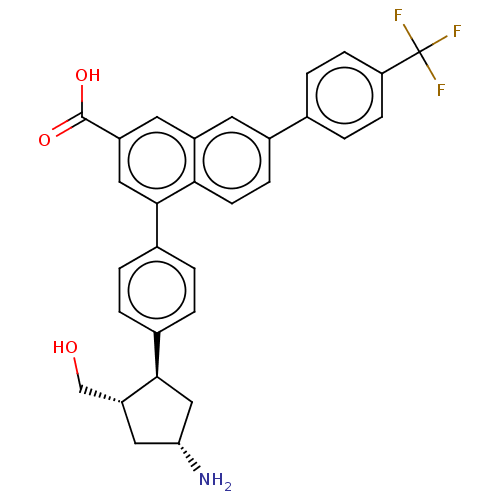 (CHEMBL5083619) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to mouse P2Y14R expressed in HEK293 cells preincubated for 30 mins followed by addition of 6-amino-9-(2-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50566405 (CHEMBL4864408) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to mouse P2Y14 receptor expressed in HEK293 cells preincubated with compound for 30 mins followed by flu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00164 BindingDB Entry DOI: 10.7270/Q2BZ69SP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343884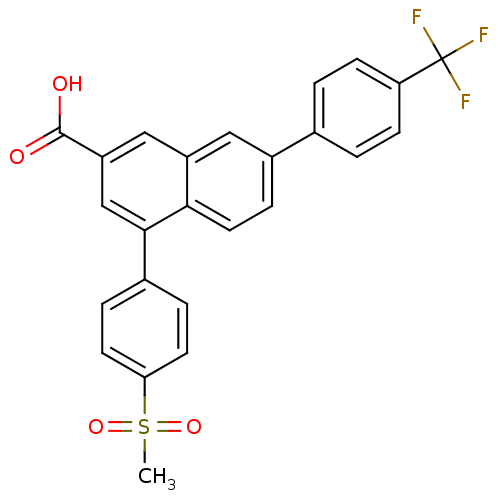 (4-(4-(methylsulfonyl)phenyl)-7-(4-(trifluoromethyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50543257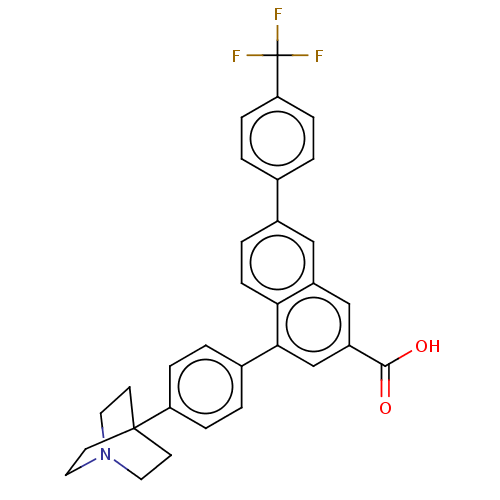 (CHEMBL4645216) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of MRS4174 binding to mouse P2Y14R expressed in HEK293 cells pre-incubated for 30 mins before MRS4174 addition and further incubated for 3... | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of MRS4174 binding to mouse P2Y14R expressed in HEK293 cells pre-incubated for 30 mins before MRS4174 addition and further incubated for 3... | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Displacement of Alexafluor488 labeled 4-(4-(1-(4-(1-(6-(4-(6-amino-3-imino-4,5-disulfo-3H-xanthen-9-yl)-3-carboxybenzamido)hexyl)-1H-1,2,3-triazol-4-... | ACS Med Chem Lett 11: 1281-1286 (2020) Article DOI: 10.1021/acsmedchemlett.0c00115 BindingDB Entry DOI: 10.7270/Q2222Z99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343095 (CHEMBL1771452 | N-(3-ethylphenyl)-2-(4-fluoropheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343876 (7-(2,6-dimethylbenzyloxy)-4-(thiophen-3-yl)-2-naph...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50584894 (CHEMBL5076127) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to mouse P2Y14R expressed in HEK293 cells preincubated for 30 mins followed by addition of 6-amino-9-(2-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343109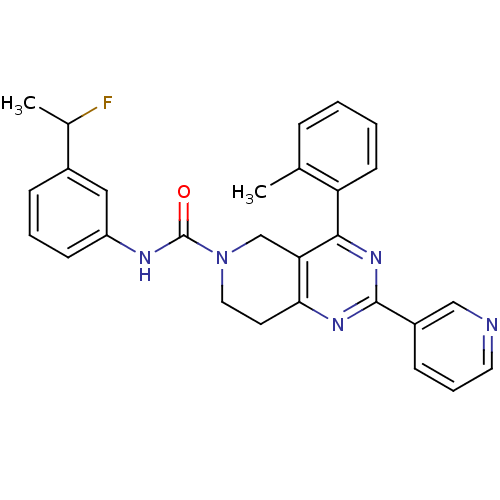 (CHEMBL1771244 | N-(3-(1-fluoroethyl)phenyl)-2-(pyr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50541998 (CHEMBL4642592) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of MRS4174 binding to mouse P2Y14R expressed in HEK293 cells pre-incubated for 30 mins before MRS4174 addition and further incubated for 3... | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343125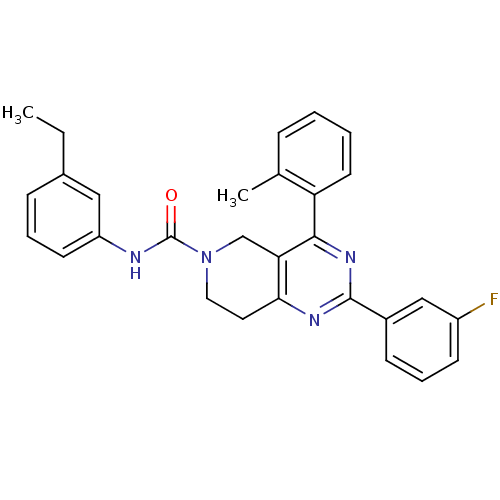 (CHEMBL1771453 | N-(3-ethylphenyl)-2-(3-fluoropheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50541998 (CHEMBL4642592) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Displacement of Alexafluor488 labeled 4-(4-(1-(4-(1-(6-(4-(6-amino-3-imino-4,5-disulfo-3H-xanthen-9-yl)-3-carboxybenzamido)hexyl)-1H-1,2,3-triazol-4-... | ACS Med Chem Lett 11: 1281-1286 (2020) Article DOI: 10.1021/acsmedchemlett.0c00115 BindingDB Entry DOI: 10.7270/Q2222Z99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50584891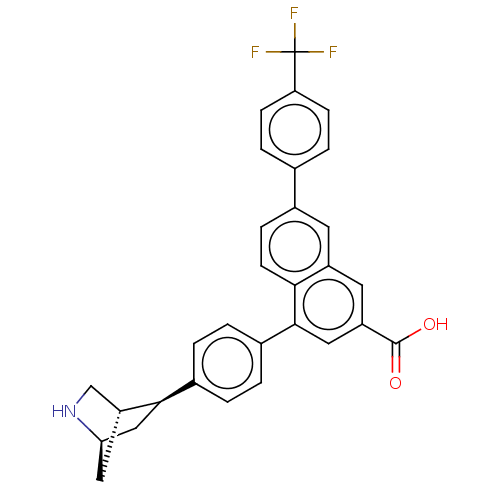 (CHEMBL5085823) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to mouse P2Y14R expressed in HEK293 cells preincubated for 30 mins followed by addition of 6-amino-9-(2-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50541997 (CHEMBL4649855) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Displacement of Alexafluor488 labeled 4-(4-(1-(4-(1-(6-(4-(6-amino-3-imino-4,5-disulfo-3H-xanthen-9-yl)-3-carboxybenzamido)hexyl)-1H-1,2,3-triazol-4-... | ACS Med Chem Lett 11: 1281-1286 (2020) Article DOI: 10.1021/acsmedchemlett.0c00115 BindingDB Entry DOI: 10.7270/Q2222Z99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50584895 (CHEMBL5076678) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to mouse P2Y14R expressed in HEK293 cells preincubated for 30 mins followed by addition of 6-amino-9-(2-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50584889 (CHEMBL5083773) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to mouse P2Y14R expressed in HEK293 cells preincubated for 30 mins followed by addition of 6-amino-9-(2-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343121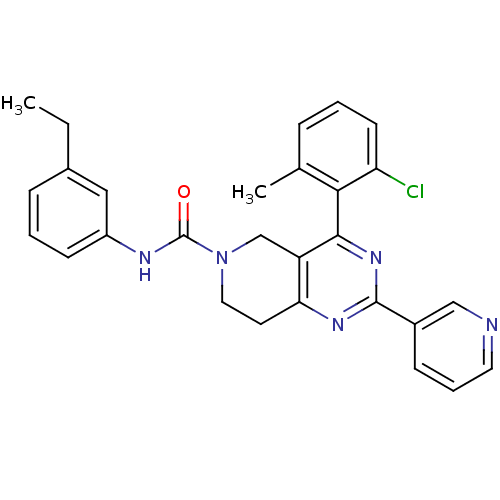 (4-(2-chloro-6-methylphenyl)-N-(3-ethylphenyl)-2-(p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343122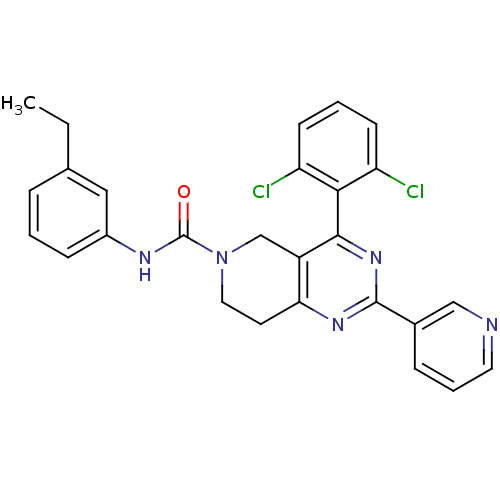 (4-(2,6-dichlorophenyl)-N-(3-ethylphenyl)-2-(pyridi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343887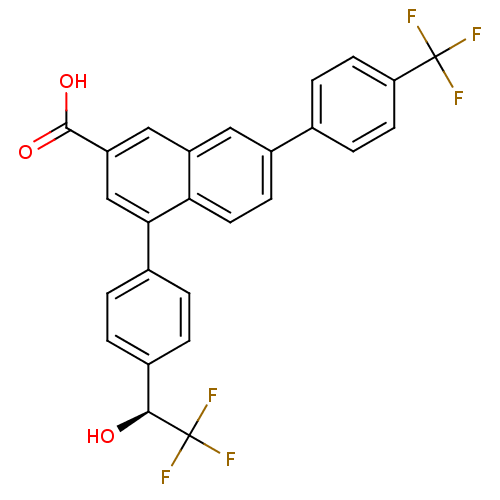 ((S)-4-(4-(2,2,2-trifluoro-1-hydroxyethyl)phenyl)-7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343878 (4-(thiophen-3-yl)-7-(4-(trifluoromethoxy)phenyl)-2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50543255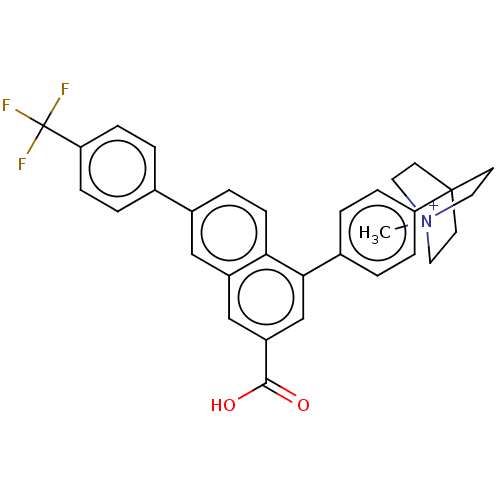 (CHEMBL4640370) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of MRS4174 binding to mouse P2Y14R expressed in HEK293 cells pre-incubated for 30 mins before MRS4174 addition and further incubated for 3... | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343090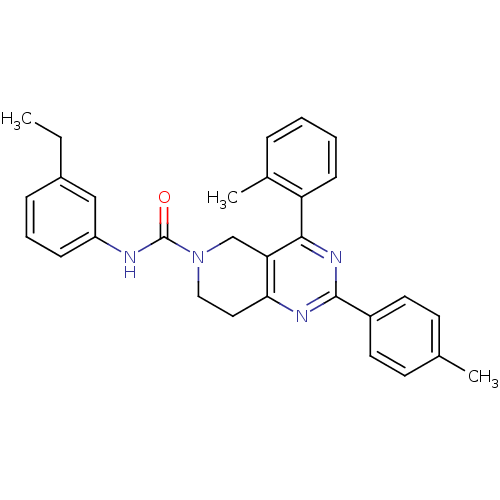 (CHEMBL1771446 | N-(3-ethylphenyl)-4-o-tolyl-2-p-to...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343103 (CHEMBL1771238 | N-(3-ethylphenyl)-2-(pyridin-3-yl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343089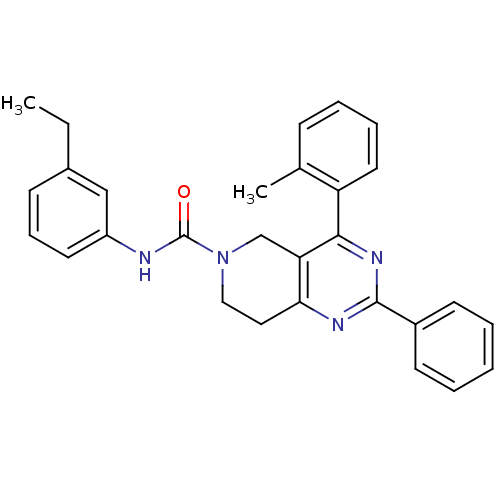 (CHEMBL1771445 | N-(3-ethylphenyl)-2-phenyl-4-o-tol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50584896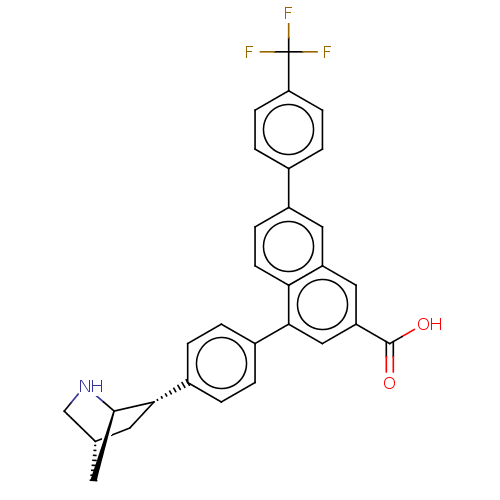 (CHEMBL5087295) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to mouse P2Y14R expressed in HEK293 cells preincubated for 30 mins followed by addition of 6-amino-9-(2-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50584904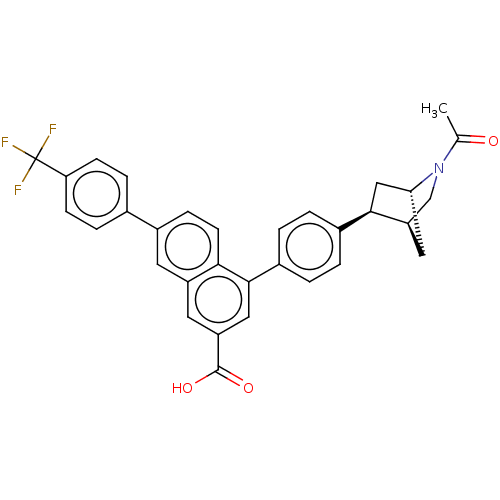 (CHEMBL5093465) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to mouse P2Y14R expressed in HEK293 cells preincubated for 30 mins followed by addition of 6-amino-9-(2-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50584886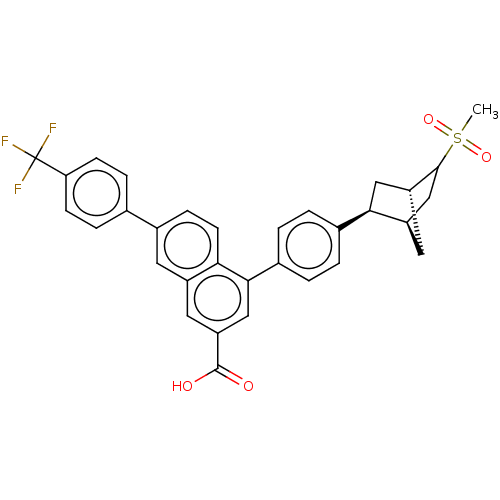 (CHEMBL5090079) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to mouse P2Y14R expressed in HEK293 cells preincubated for 30 mins followed by addition of 6-amino-9-(2-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343875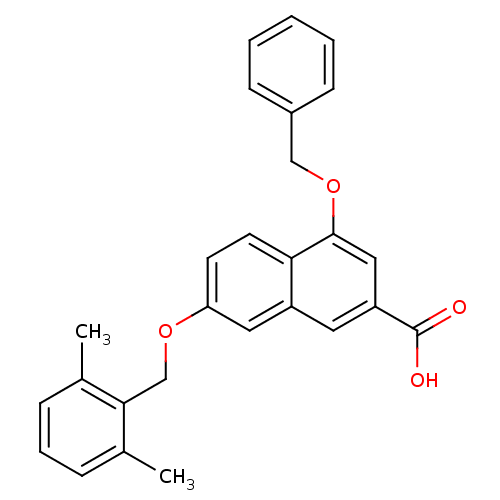 (4-(benzyloxy)-7-(2,6-dimethylbenzyloxy)-2-naphthoi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343872 (7-(2,6-dimethylbenzyloxy)-4-phenyl-2-naphthoic aci...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50456170 (CHEMBL4212441) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of MRS4174 binding to mouse P2Y14R expressed in HEK293 cells pre-incubated for 30 mins before MRS4174 addition and further incubated for 3... | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50456170 (CHEMBL4212441) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Displacement of Alexafluor488 labeled 4-(4-(1-(4-(1-(6-(4-(6-amino-3-imino-4,5-disulfo-3H-xanthen-9-yl)-3-carboxybenzamido)hexyl)-1H-1,2,3-triazol-4-... | ACS Med Chem Lett 11: 1281-1286 (2020) Article DOI: 10.1021/acsmedchemlett.0c00115 BindingDB Entry DOI: 10.7270/Q2222Z99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 104 total ) | Next | Last >> |