

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasminogen (Mus musculus) | BDBM50499241 (CHEMBL3735513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University Curated by ChEMBL | Assay Description Inhibition of mouse plasmin after 15 mins using H-D-Ile-Pro-Arg-p-nitroanilide as substrate | J Med Chem 58: 8868-76 (2015) Article DOI: 10.1021/acs.jmedchem.5b01128 BindingDB Entry DOI: 10.7270/Q2XK8JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Mus musculus) | BDBM50499237 (CHEMBL3735217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University Curated by ChEMBL | Assay Description Inhibition of mouse plasmin after 15 mins using H-D-Ile-Pro-Arg-p-nitroanilide as substrate | J Med Chem 58: 8868-76 (2015) Article DOI: 10.1021/acs.jmedchem.5b01128 BindingDB Entry DOI: 10.7270/Q2XK8JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Mus musculus) | BDBM50499238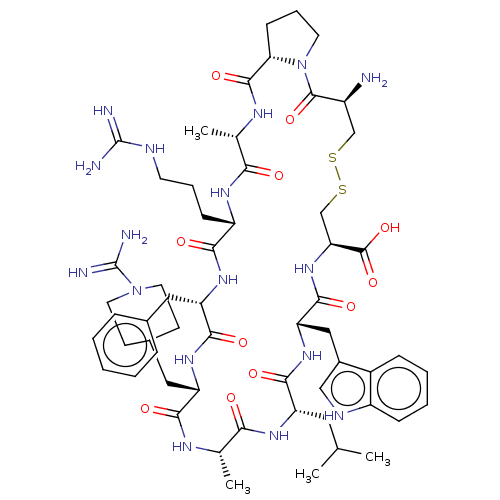 (CHEMBL3735263) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University Curated by ChEMBL | Assay Description Inhibition of mouse plasmin after 15 mins using H-D-Ile-Pro-Arg-p-nitroanilide as substrate | J Med Chem 58: 8868-76 (2015) Article DOI: 10.1021/acs.jmedchem.5b01128 BindingDB Entry DOI: 10.7270/Q2XK8JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Mus musculus) | BDBM50531924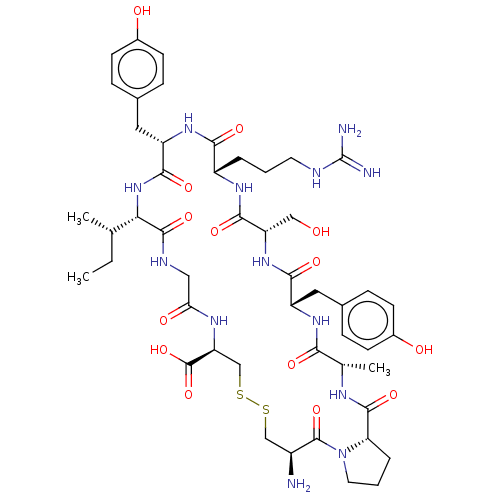 (CHEMBL4464577) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse plasmin preincubated for 15 mins followed by chromogenic substrate addition | J Med Chem 62: 2172-2183 (2019) Article DOI: 10.1021/acs.jmedchem.8b01908 BindingDB Entry DOI: 10.7270/Q24F1V6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Mus musculus) | BDBM50531926 (CHEMBL4550668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse plasmin preincubated for 15 mins followed by chromogenic substrate addition | J Med Chem 62: 2172-2183 (2019) Article DOI: 10.1021/acs.jmedchem.8b01908 BindingDB Entry DOI: 10.7270/Q24F1V6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Mus musculus) | BDBM50531925 (CHEMBL4522772) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse plasmin preincubated for 15 mins followed by chromogenic substrate addition | J Med Chem 62: 2172-2183 (2019) Article DOI: 10.1021/acs.jmedchem.8b01908 BindingDB Entry DOI: 10.7270/Q24F1V6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Mus musculus) | BDBM50499240 (CHEMBL3735080) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University Curated by ChEMBL | Assay Description Inhibition of mouse plasmin after 15 mins using H-D-Ile-Pro-Arg-p-nitroanilide as substrate | J Med Chem 58: 8868-76 (2015) Article DOI: 10.1021/acs.jmedchem.5b01128 BindingDB Entry DOI: 10.7270/Q2XK8JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Mus musculus) | BDBM50499239 (CHEMBL3734777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University Curated by ChEMBL | Assay Description Inhibition of mouse plasmin after 15 mins using H-D-Ile-Pro-Arg-p-nitroanilide as substrate | J Med Chem 58: 8868-76 (2015) Article DOI: 10.1021/acs.jmedchem.5b01128 BindingDB Entry DOI: 10.7270/Q2XK8JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264615 (US9718760, C182) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264650 (US9718760, C204) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264630 (US9718760, C198) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264611 (US9718760, C188) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264602 (US9718760, C197) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 173 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264616 (US9718760, C183) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264613 (US9718760, C157) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264609 (US9718760, C163) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 288 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264610 (US9718760, C165) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264645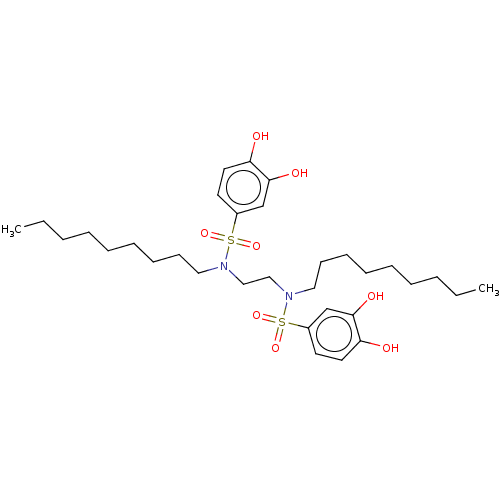 (US9718760, C185) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 420 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264646 (US9718760, C196) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 450 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM264610 (US9718760, C165) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264644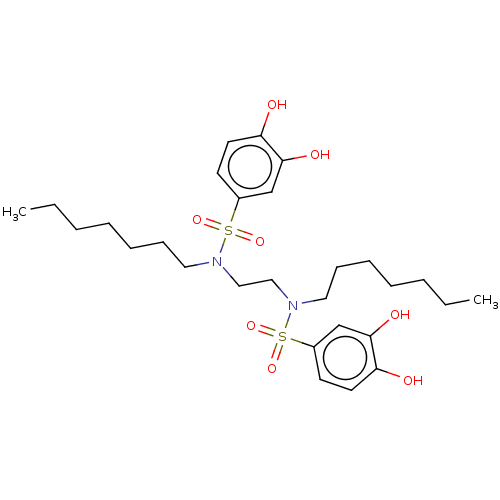 (US9718760, C184) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 590 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM264609 (US9718760, C163) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 611 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264643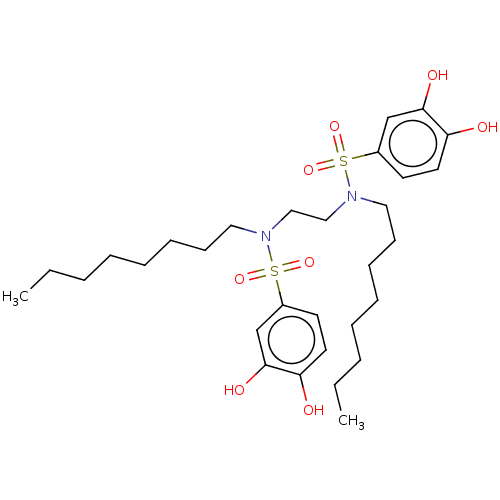 (US9718760, C176) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264607 (US9718760, C153) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264628 (US9718760, C187) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 910 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264612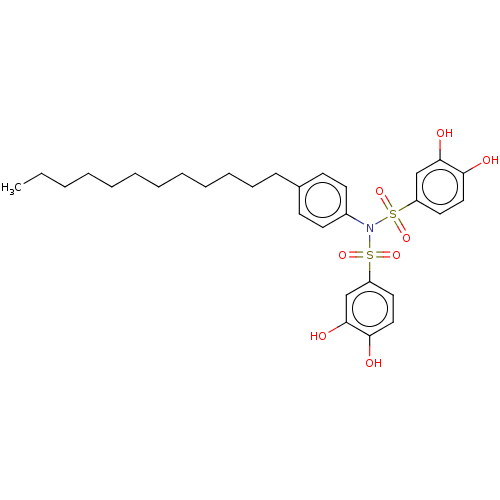 (US9718760, C195) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 920 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM264613 (US9718760, C157) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM264607 (US9718760, C153) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264601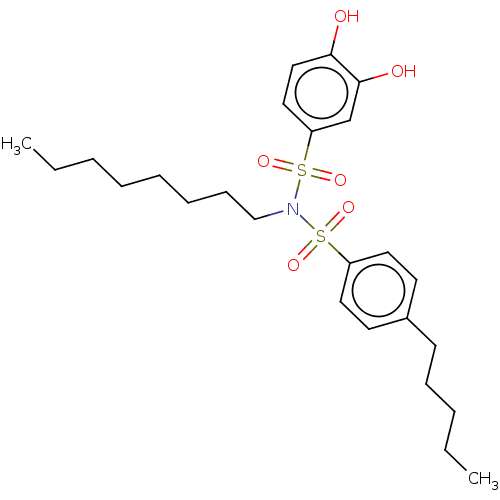 (US9718760, C191) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264623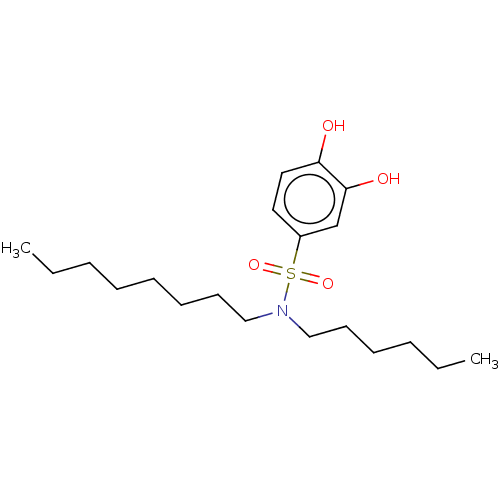 (US9718760, C180) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM264614 (US9718760, C158) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264597 (US9718760, C152) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Mus musculus) | BDBM50467212 (CHEMBL4285028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdulaziz University for Health Sciences Curated by ChEMBL | Assay Description Inhibition of mouse plasmin | Bioorg Med Chem Lett 28: 3372-3375 (2018) Article DOI: 10.1016/j.bmcl.2018.09.001 BindingDB Entry DOI: 10.7270/Q24Q7XNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264618 (US9718760, C171) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM264597 (US9718760, C152) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264635 (US9718760, C271) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264619 (US9718760, C172) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264642 (US9718760, C168) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM264648 (US9718760, C161) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description PAI-1 inhibitor compounds were dissolved in DMSO to a final concentration of (10-50 mM), depending upon solubility. Compounds were then diluted in ph... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264600 (US9718760, C189) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264622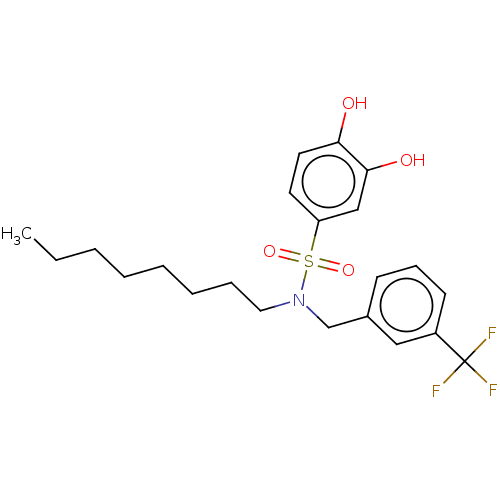 (US9718760, C179) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264614 (US9718760, C158) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264648 (US9718760, C161) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264608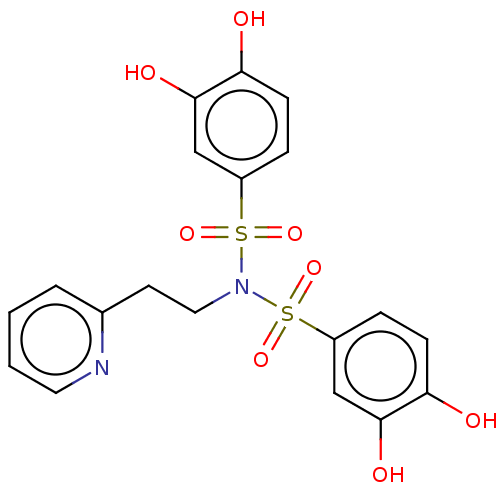 (US9718760, C162) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264603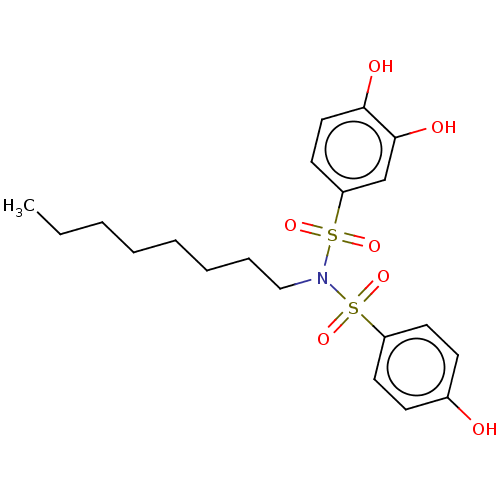 (US9718760, C224) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Mus musculus) | BDBM50228412 (CHEMBL393979 | methyl 1-(bis(4-acetamidophenoxy)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of mouse plasmin | J Med Chem 50: 6638-46 (2007) Article DOI: 10.1021/jm700962j BindingDB Entry DOI: 10.7270/Q2MW2J0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264660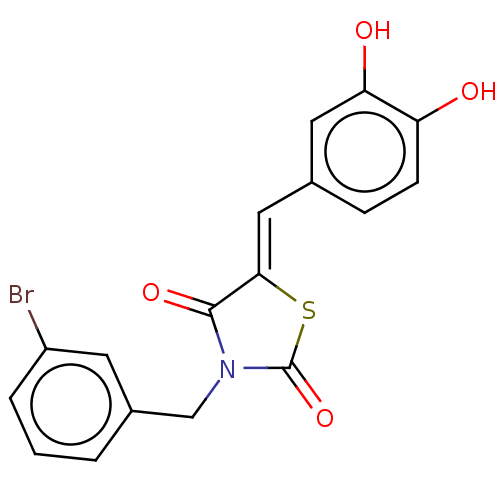 (US9718760, C206) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.96E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264626 (US9718760, C205) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.05E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264621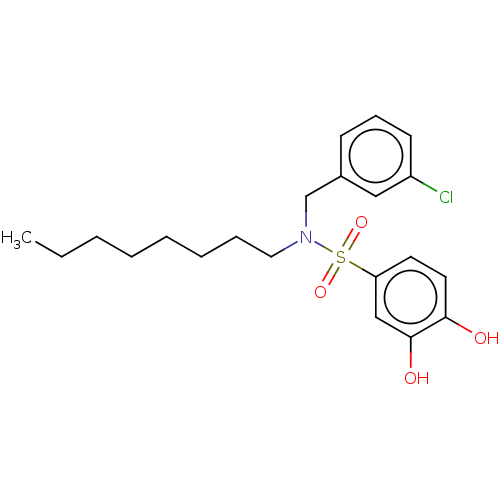 (US9718760, C177) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.11E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1/Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM264658 (US9718760, C199) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.36E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized compounds as PAI-1 inhibitors, a fluorometric plate assay was carried out to measure the half maxima... | US Patent US9718760 (2017) BindingDB Entry DOI: 10.7270/Q2K939HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 262 total ) | Next | Last >> |