

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM199180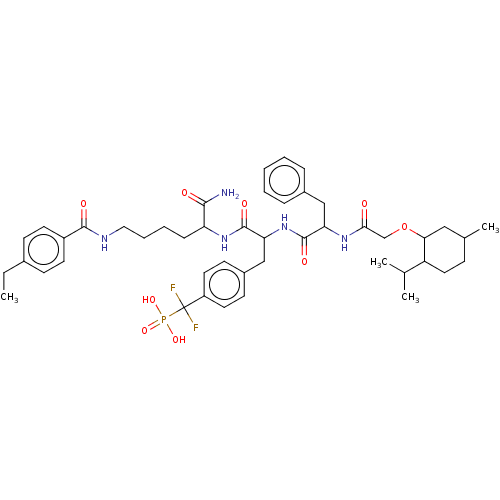 (US9217012, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-34.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40777 (Hemicalyculin A (5)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40787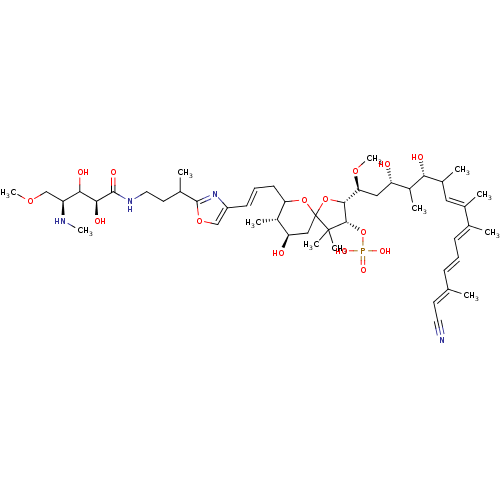 (Des-N-methylcalyculin A (20)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40776 (Calyculin A (4) | Calyculin B (19) | Calyculin E (...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40782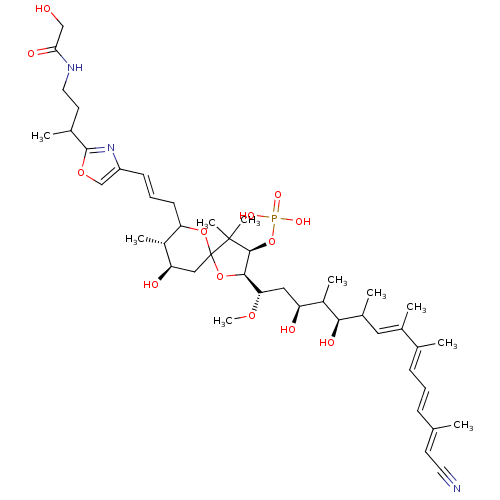 (C1/C34-Calyculin A (15)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40785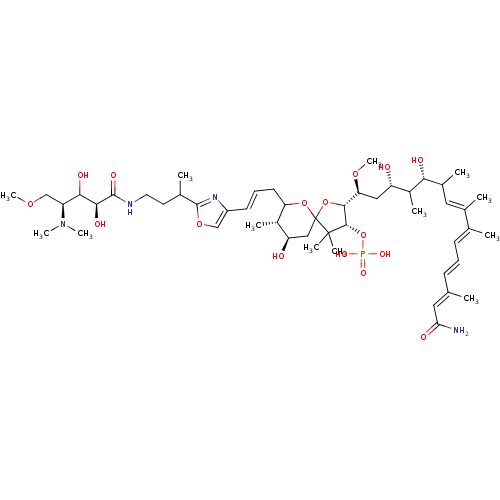 (Calyculinamide A (18)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40780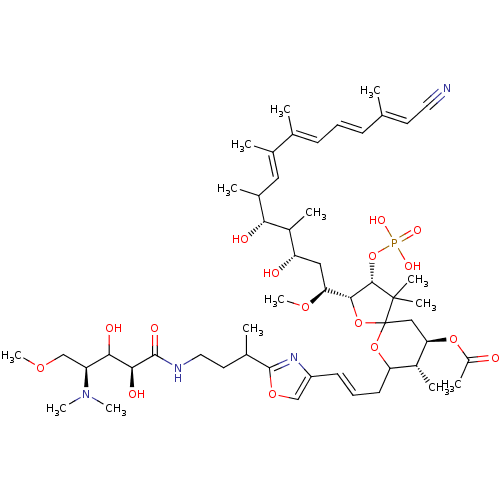 (Calyculin A 21-acetate (13)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40790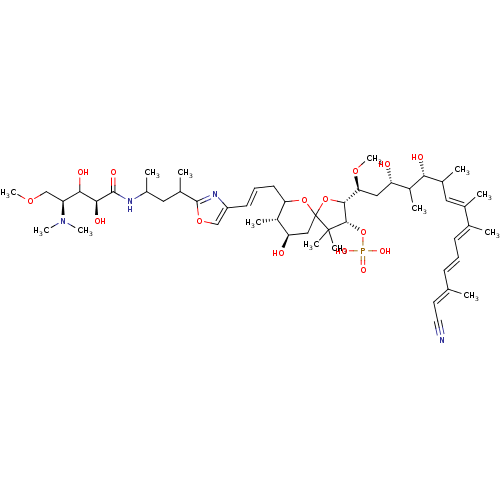 (Calyculin C (24)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40776 (Calyculin A (4) | Calyculin B (19) | Calyculin E (...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40776 (Calyculin A (4) | Calyculin B (19) | Calyculin E (...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40776 (Calyculin A (4) | Calyculin B (19) | Calyculin E (...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40781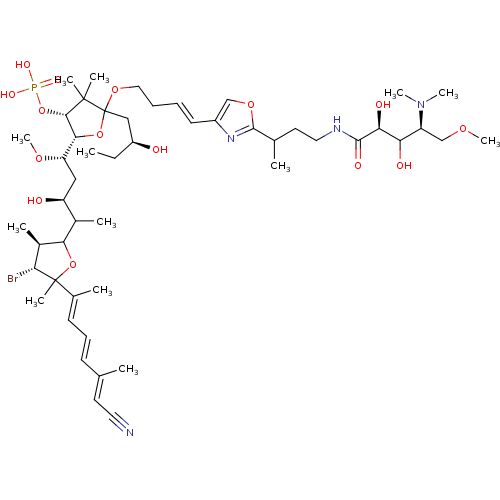 (Calyculin J (14)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50127377 (10-[3,9-dimethyl-8-(3-methyl-4-oxopentyl)-(9R)-1,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibition of protein phosphatase 2A (PP2A) was determined by standard phosphorylase a inhibition assay | Bioorg Med Chem Lett 13: 1597-600 (2003) BindingDB Entry DOI: 10.7270/Q2DB82CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50127375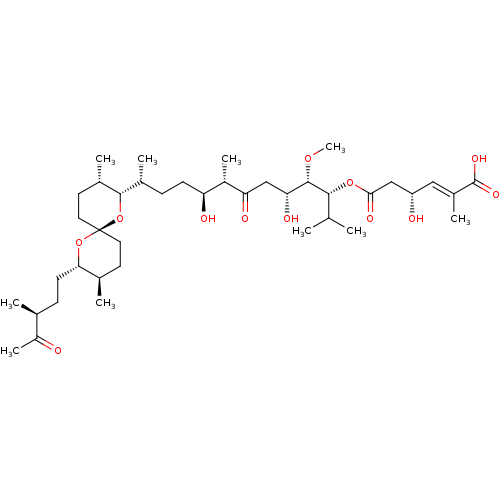 ((E)-(R)-4-Hydroxy-2-methyl-hex-2-enedioic acid 6-{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibition of protein phosphatase 2A (PP2A) was determined by standard phosphorylase a inhibition assay | Bioorg Med Chem Lett 13: 1597-600 (2003) BindingDB Entry DOI: 10.7270/Q2DB82CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50366883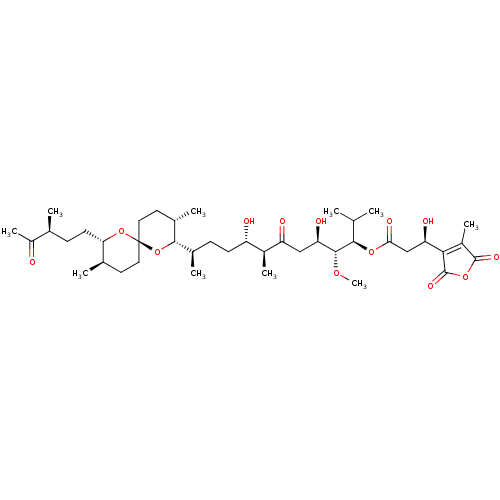 (TAUTOMYCIN) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibition of protein phosphatase 2A (PP2A) was determined by standard phosphorylase a inhibition assay | Bioorg Med Chem Lett 13: 1597-600 (2003) BindingDB Entry DOI: 10.7270/Q2DB82CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40783 (C9/C35-calyculin (16)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40779 (Calyculin A 11,13,21-triacetate (12)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40778 (11,13-O-isopropylidene-calyculin A (6)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50222937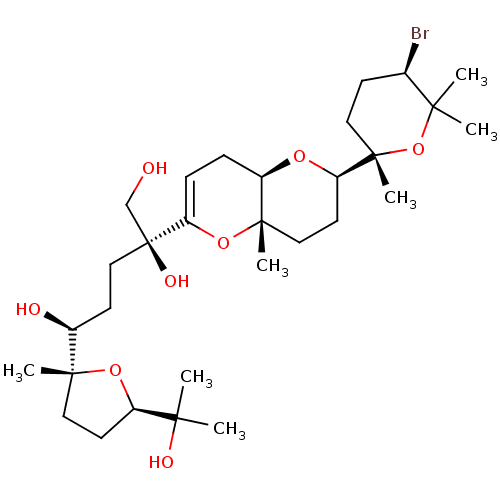 (CHEMBL23732) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Universitario de Bio-Org£nica Curated by ChEMBL | Assay Description Inhibitory activity against protein phosphatase (PP2A) using fluorescein diphosphate as substrate | Bioorg Med Chem Lett 13: 1261-4 (2003) BindingDB Entry DOI: 10.7270/Q2DR2XPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50222936 (CHEMBL18962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Universitario de Bio-Org£nica Curated by ChEMBL | Assay Description Inhibitory activity against protein phosphatase (PP2A) using fluorescein diphosphate as substrate | Bioorg Med Chem Lett 13: 1261-4 (2003) BindingDB Entry DOI: 10.7270/Q2DR2XPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM40784 (Dephosphonocalyculin A (17)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo | Assay Description Protein phosphatase inhibitory activity of calyculin derivatives. | Chem Biol 9: 309-19 (2002) Article DOI: 10.1016/S1074-5521(02)00118-7 BindingDB Entry DOI: 10.7270/Q2W66J5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50307997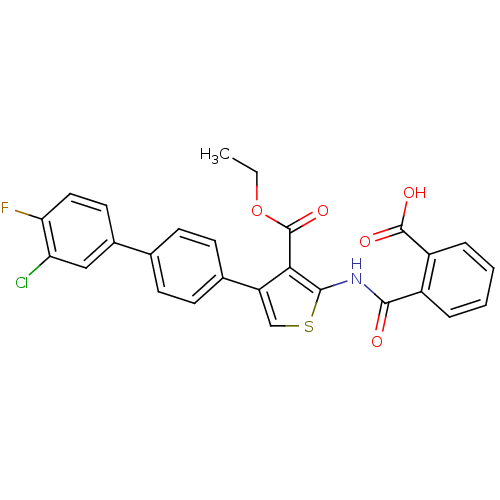 (2-(2-Carboxy-benzoylamino)-4-(30-chloro-40-fluorob...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of PTPalpha expressed in Escherichia coli BL21 (DE3) after 10 mins by spectrophotometry | Bioorg Med Chem 18: 1773-82 (2010) Article DOI: 10.1016/j.bmc.2010.01.055 BindingDB Entry DOI: 10.7270/Q2X06811 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50127378 ((S)-2-Hydroxy-2-methyl-succinic acid 4-{(1R,2S,3R,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibition of protein phosphatase 2A (PP2A) was determined by standard phosphorylase a inhibition assay | Bioorg Med Chem Lett 13: 1597-600 (2003) BindingDB Entry DOI: 10.7270/Q2DB82CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50544431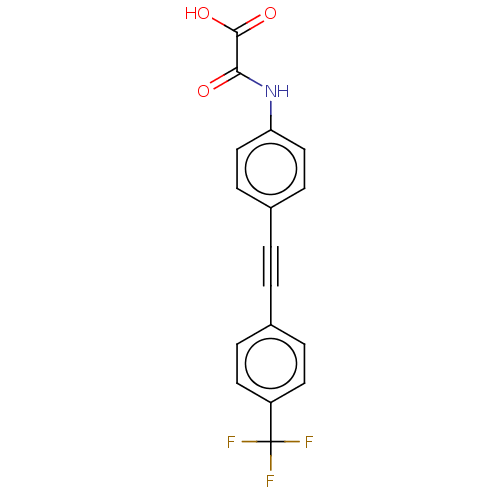 (CHEMBL4637459 | US11192850, Entry 4k) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of PTPA (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50544440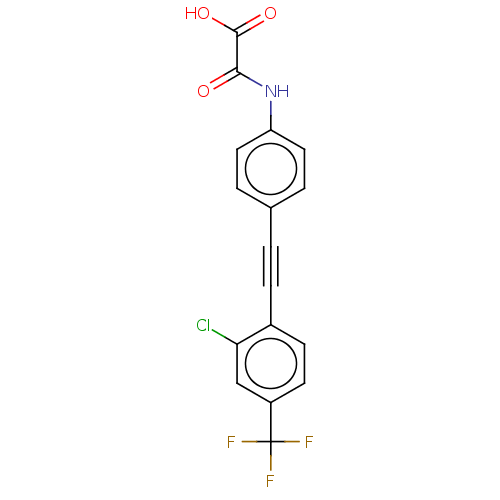 (CHEMBL4647367 | US11192850, Entry 4t) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of PTPA (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50307999 (4-Biphenyl-4-yl-2-(2-carboxy-benzoylamino)-5-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of PTPalpha expressed in Escherichia coli BL21 (DE3) after 10 mins by spectrophotometry | Bioorg Med Chem 18: 1773-82 (2010) Article DOI: 10.1016/j.bmc.2010.01.055 BindingDB Entry DOI: 10.7270/Q2X06811 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50544427 (CHEMBL4632818 | US11192850, Entry 4g) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of PTPA (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50307990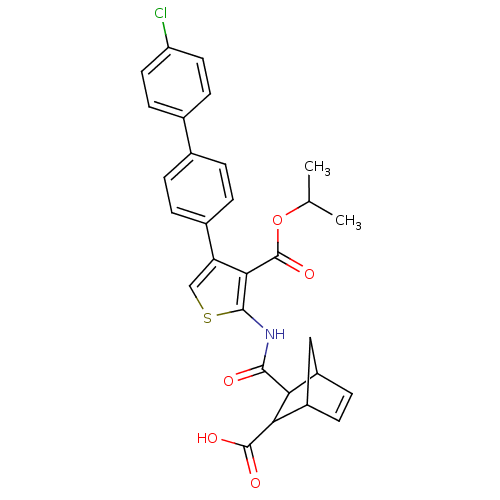 (2-[(3-Carboxy-bicyclo[2.2.1]hept-5-ene-2-carbonyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of PTPalpha expressed in Escherichia coli BL21 (DE3) after 10 mins by spectrophotometry | Bioorg Med Chem 18: 1773-82 (2010) Article DOI: 10.1016/j.bmc.2010.01.055 BindingDB Entry DOI: 10.7270/Q2X06811 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50307989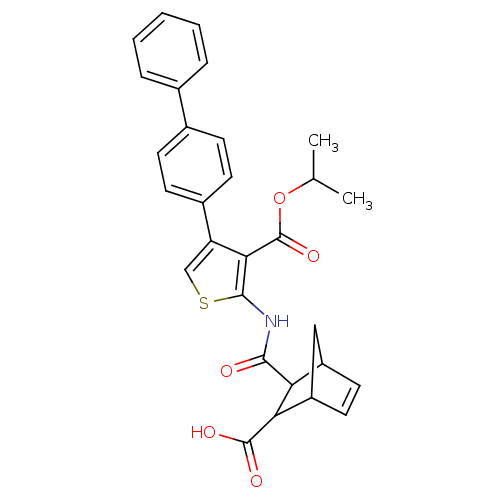 (4-Biphenyl-4-yl-2-[(3-carboxy-bicyclo[2.2.1]hept-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of PTPalpha expressed in Escherichia coli BL21 (DE3) after 10 mins by spectrophotometry | Bioorg Med Chem 18: 1773-82 (2010) Article DOI: 10.1016/j.bmc.2010.01.055 BindingDB Entry DOI: 10.7270/Q2X06811 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50308000 (3-(3,5-dibromo-4-hydroxybenzoyl)-2-ethyl-N-(thiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of PTPalpha expressed in Escherichia coli BL21 (DE3) after 10 mins by spectrophotometry | Bioorg Med Chem 18: 1773-82 (2010) Article DOI: 10.1016/j.bmc.2010.01.055 BindingDB Entry DOI: 10.7270/Q2X06811 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50127376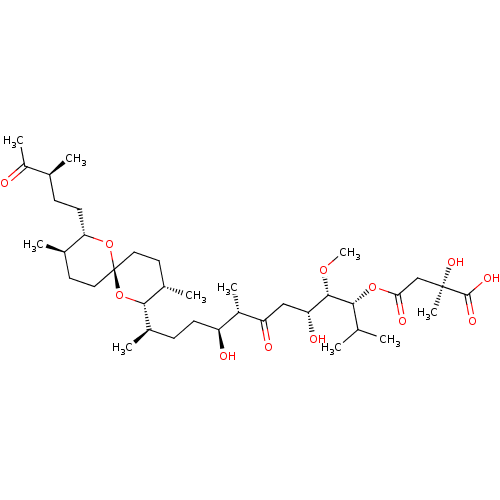 ((R)-2-Hydroxy-2-methyl-succinic acid 4-{(1R,2S,3R,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibition of protein phosphatase 2A (PP2A) was determined by standard phosphorylase a inhibition assay | Bioorg Med Chem Lett 13: 1597-600 (2003) BindingDB Entry DOI: 10.7270/Q2DB82CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2A activator (Homo sapiens (Human)) | BDBM50127379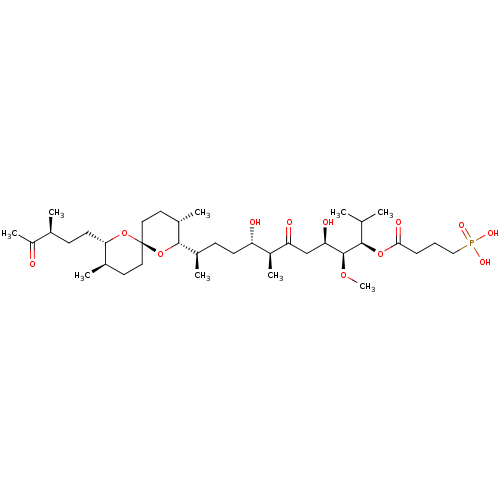 (4-Phosphono-butyric acid (1R,2S,3R,6S,7S,10R)-10-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibition of protein phosphatase 2A (PP2A) was determined by standard phosphorylase a inhibition assay | Bioorg Med Chem Lett 13: 1597-600 (2003) BindingDB Entry DOI: 10.7270/Q2DB82CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||