

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21283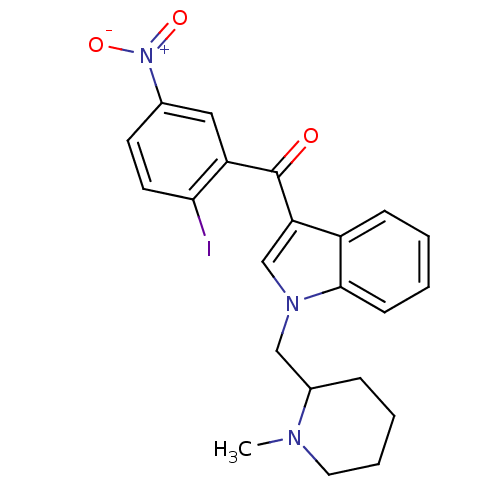 (3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM21283 (3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 216 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity at rat CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation | Cell Chem Biol 56: 8224-56 (2013) Article DOI: 10.1021/jm4005626 BindingDB Entry DOI: 10.7270/Q2B859M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM21283 (3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 216 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity at rat CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation | Cell Chem Biol 56: 8224-56 (2013) Article DOI: 10.1021/jm4005626 BindingDB Entry DOI: 10.7270/Q2B859M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21283 (3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21283 (3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 11.5 | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 51: 1904-12 (2008) Article DOI: 10.1021/jm7011613 BindingDB Entry DOI: 10.7270/Q2C827K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21283 (3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in HEK293 cells coexpressing Galphaq/o5 assessed as calcium mobilization by FLIPR assay | J Med Chem 53: 295-315 (2010) Article DOI: 10.1021/jm901214q BindingDB Entry DOI: 10.7270/Q2KD1Z00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||