

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000780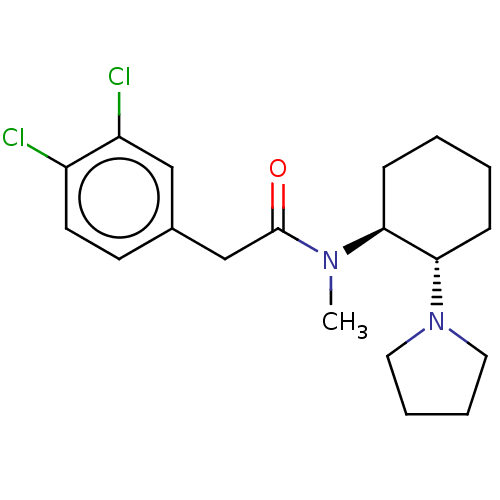 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Activity at kappa opioid receptor expressed in HEK293 cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000780 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membrane incubated for 1 hr by [35S]GTPgammaS binding based liquid scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01082 BindingDB Entry DOI: 10.7270/Q2NZ8CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50000780 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center Curated by ChEMBL | Assay Description Agonist activity at mouse kappa opioid receptor-1 expressed in CHO cell membranes assessed as [35S]GTPgammaS binding incubated for 60 mins by scintil... | J Med Chem 59: 8381-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00748 BindingDB Entry DOI: 10.7270/Q27H1P2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000780 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by [35S]GTPgammaS binding assay | J Med Chem 63: 7663-7694 (2020) Article DOI: 10.1021/acs.jmedchem.0c00503 BindingDB Entry DOI: 10.7270/Q2B56P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000780 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells | J Med Chem 49: 256-62 (2006) Article DOI: 10.1021/jm050577x BindingDB Entry DOI: 10.7270/Q2HQ40QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000780 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 54: 1903-13 (2011) Article DOI: 10.1021/jm101542c BindingDB Entry DOI: 10.7270/Q2028SJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||