

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50012973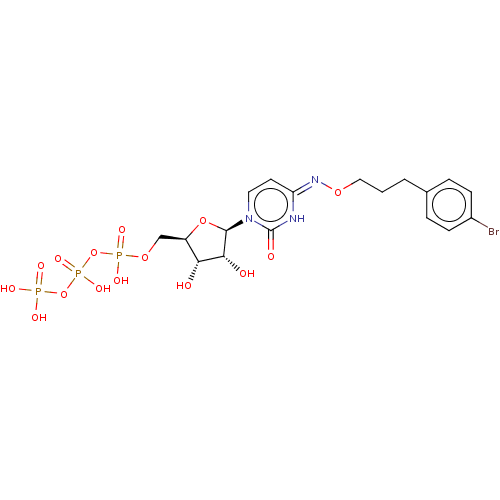 (CHEMBL3261364) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 141 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y4 receptor expressed in human 1321N1 cells assessed as stimulation of [3H]inositol phosphate accumulation by liquid scin... | J Med Chem 57: 3874-83 (2014) Article DOI: 10.1021/jm500367e BindingDB Entry DOI: 10.7270/Q2V989M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50012973 (CHEMBL3261364) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 225 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells assessed as stimulation of [3H]inositol phosphate accumulation by liquid scin... | J Med Chem 57: 3874-83 (2014) Article DOI: 10.1021/jm500367e BindingDB Entry DOI: 10.7270/Q2V989M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50012973 (CHEMBL3261364) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in human 1321N1 cells assessed as stimulation of [3H]inositol phosphate accumulation by liquid scin... | J Med Chem 57: 3874-83 (2014) Article DOI: 10.1021/jm500367e BindingDB Entry DOI: 10.7270/Q2V989M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||