

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101823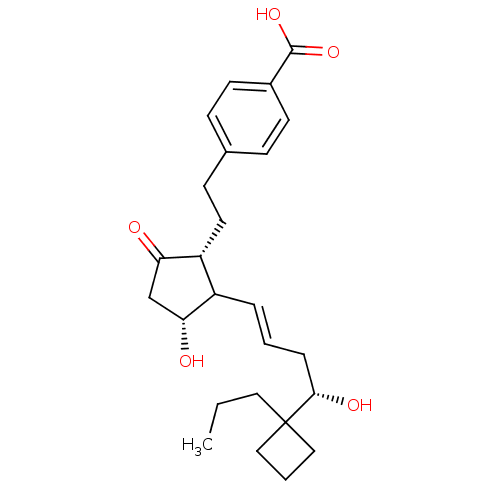 (4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP2 receptor | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101823 (4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP2 receptor | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||