

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50118810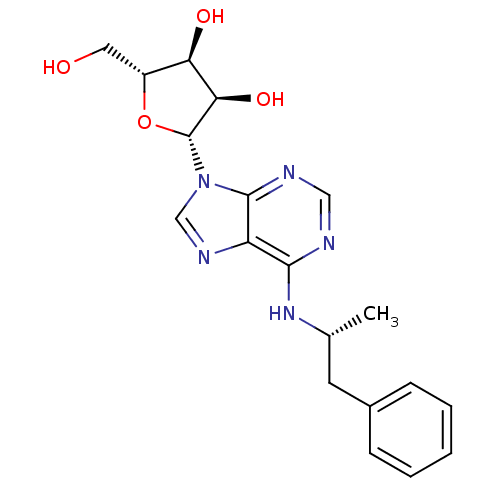 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effect on forskolin-stimulated cyclic AMP production in intact chinese hamster ovary (CHO) cell expressing the human Adenosine A3 receptor | J Med Chem 45: 4471-84 (2002) BindingDB Entry DOI: 10.7270/Q2P84CMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Homo sapiens (Human)) | BDBM50118810 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 950 | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition of Adenylate cyclase activity in rat fat cell membrane at adenosine A1 receptor | J Med Chem 31: 1179-83 (1988) BindingDB Entry DOI: 10.7270/Q2QC042G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50118810 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells | J Nat Prod 69: 432-5 (2006) Article DOI: 10.1021/np058114h BindingDB Entry DOI: 10.7270/Q2V98907 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50118810 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of cAMP production | J Med Chem 50: 1810-27 (2007) Article DOI: 10.1021/jm061278q BindingDB Entry DOI: 10.7270/Q28916P1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50118810 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.96E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as stimulation of adenylate cyclase | J Med Chem 51: 2088-99 (2008) Article DOI: 10.1021/jm701442d BindingDB Entry DOI: 10.7270/Q2R49RNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||