

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50170678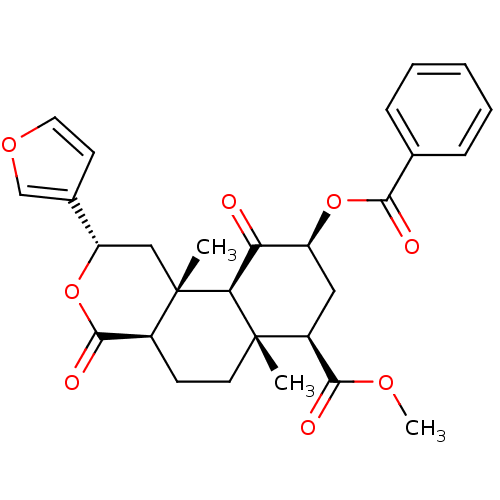 ((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human mu opiod receptor expressed in CHO-K1 cells assessed as increase in forskolin induced cAMP production measured after 30 min... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b01235 BindingDB Entry DOI: 10.7270/Q2FJ2MH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50170678 ((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human kappa opiod receptor expressed in CHO-K1 cells assessed as increase in forskolin induced cAMP production measured after 30 ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b01235 BindingDB Entry DOI: 10.7270/Q2FJ2MH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50170678 ((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Agonist activity at MOR (unknown origin) | Bioorg Med Chem Lett 24: 4895-8 (2014) Article DOI: 10.1016/j.bmcl.2014.08.012 BindingDB Entry DOI: 10.7270/Q2C53NFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50170678 ((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Agonist activity at human recombinant mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | J Med Chem 51: 2421-31 (2008) Article DOI: 10.1021/jm701162g BindingDB Entry DOI: 10.7270/Q2DV1KR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50170678 ((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding to Opioid receptor mu1 expressed in CHO cells | J Med Chem 48: 4765-71 (2005) Article DOI: 10.1021/jm048963m BindingDB Entry DOI: 10.7270/Q2DN44KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50170678 ((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding to Opioid receptor kappa1 expressed in CHO cells | J Med Chem 48: 4765-71 (2005) Article DOI: 10.1021/jm048963m BindingDB Entry DOI: 10.7270/Q2DN44KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50170678 ((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Agonist activity at human recombinant kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | J Med Chem 51: 2421-31 (2008) Article DOI: 10.1021/jm701162g BindingDB Entry DOI: 10.7270/Q2DV1KR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50170678 ((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human mu opiod receptor expressed in CHO-K1 cells assessed as stimulation of beta-arrestin recruitment measured after 90 mins by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b01235 BindingDB Entry DOI: 10.7270/Q2FJ2MH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50170678 ((3S,4aR,4bS,6S,8R,8aR,10aR)-6-Benzoyloxy-3-furan-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human delta opiod receptor expressed in CHO-K1 cells assessed as increase in forskolin induced cAMP production measured after 30 ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b01235 BindingDB Entry DOI: 10.7270/Q2FJ2MH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||