

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177016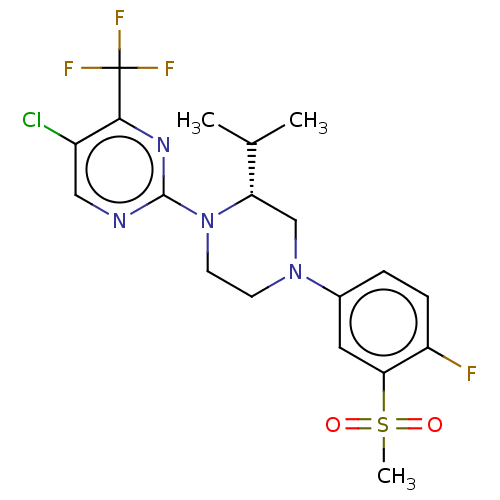 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at human LXRbeta ligand binding domain(155 to 460 residues) transfected in HEK293 cells after 16 hrs by Gal4-luciferase reporter gen... | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177016 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 92 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity at human LXRalpha ligand binding domain(167 to 447 residues) transfected in HEK293 cells after 16 hrs by Gal4-luciferase reporter ge... | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||