

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM50301375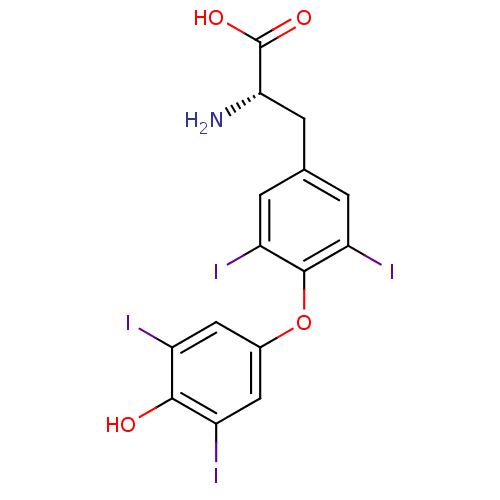 (3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Agonist activity at THRalpha (unknown origin) incubated for 14 to 16 hrs by dual glo luciferase reporter gene assay | J Med Chem 63: 6727-6740 (2020) Article DOI: 10.1021/acs.jmedchem.9b02150 BindingDB Entry DOI: 10.7270/Q23N26ZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50301375 (3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Agonist activity at THRbeta (unknown origin) incubated for 14 to 16 hrs by dual glo luciferase reporter gene assay | J Med Chem 63: 6727-6740 (2020) Article DOI: 10.1021/acs.jmedchem.9b02150 BindingDB Entry DOI: 10.7270/Q23N26ZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50301375 (3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50301375 (3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Agonist activity at recombinant human pFA-CMV fused PPARgamma expressed in HEK293T cells transfected with pFR-luciferase plasmid and pRL-SV40 plasmid... | J Med Chem 63: 6727-6740 (2020) Article DOI: 10.1021/acs.jmedchem.9b02150 BindingDB Entry DOI: 10.7270/Q23N26ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50301375 (3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Agonist activity at recombinant human pFA-CMV fused RXRalpha LBD expressed in HEK293T cells transfected with pFR-luciferase plasmid and pRL-SV40 plas... | J Med Chem 63: 6727-6740 (2020) Article DOI: 10.1021/acs.jmedchem.9b02150 BindingDB Entry DOI: 10.7270/Q23N26ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50301375 (3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||