 Found 5 hits of ec50 for monomerid = 8885
Found 5 hits of ec50 for monomerid = 8885 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM8885
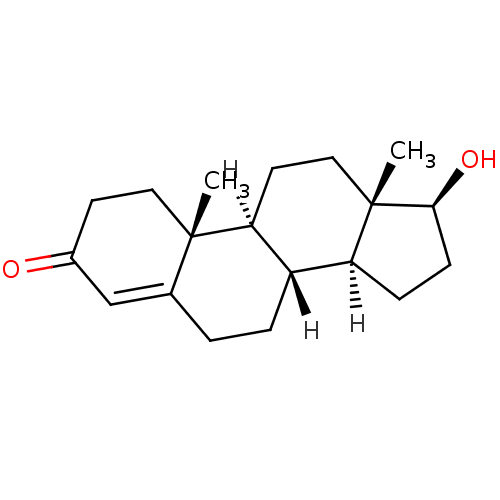
((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research& Development , 610 Main Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
| Assay Description
Androgenic activity at human androgen receptor expressed in African green monkey CV-1 cells assessed as increase in interaction between VP16-fused AR... |
J Med Chem 60: 6451-6457 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00373
BindingDB Entry DOI: 10.7270/Q2HH6NH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM8885

((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114119
BindingDB Entry DOI: 10.7270/Q2P55SHP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Mus musculus) | BDBM8885

((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... |
J Med Chem 52: 7186-91 (2009)
Article DOI: 10.1021/jm901149c
BindingDB Entry DOI: 10.7270/Q2SJ1MV9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM8885

((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor in human MDA-KB2 cells transfected with MMTV linked luciferase assessed as transcriptional activation by lucife... |
J Med Chem 52: 7186-91 (2009)
Article DOI: 10.1021/jm901149c
BindingDB Entry DOI: 10.7270/Q2SJ1MV9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM8885

((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in CHO cells by luciferase assay |
J Med Chem 51: 1831-41 (2008)
Article DOI: 10.1021/jm7015864
BindingDB Entry DOI: 10.7270/Q2222VNJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data