 Found 12 hits of ic50 for monomerid = 10692
Found 12 hits of ic50 for monomerid = 10692 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10692
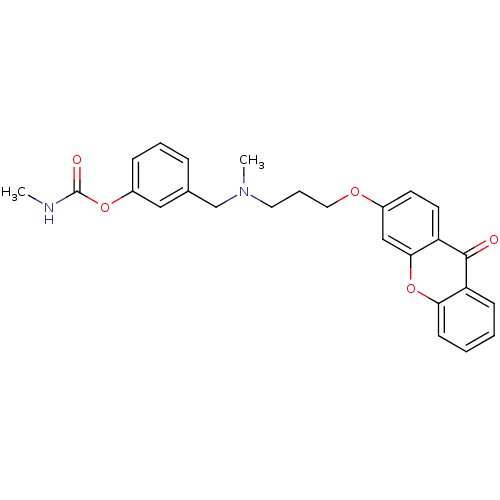
(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... |
J Med Chem 41: 3976-86 (1998)
Article DOI: 10.1021/jm9810046
BindingDB Entry DOI: 10.7270/Q2KH0KJ8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10692

(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AchE |
Bioorg Med Chem 15: 575-85 (2006)
Article DOI: 10.1016/j.bmc.2006.09.026
BindingDB Entry DOI: 10.7270/Q2JS9Q2H |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10692

(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114606
BindingDB Entry DOI: 10.7270/Q2FJ2MW1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10692

(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-010-9373-7
BindingDB Entry DOI: 10.7270/Q2X069X3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10692

(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
ACS Med Chem Lett 3: 182-186 (2012)
Article DOI: 10.1021/ml200313p
BindingDB Entry DOI: 10.7270/Q29W0GJB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10692

(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... |
J Med Chem 48: 4444-56 (2005)
Article DOI: 10.1021/jm049515h
BindingDB Entry DOI: 10.7270/Q2R78CF8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10692

(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10692

(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AchE |
J Med Chem 51: 347-72 (2008)
Article DOI: 10.1021/jm7009364
BindingDB Entry DOI: 10.7270/Q25B039W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10692

(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human serum BuChE |
Bioorg Med Chem 15: 575-85 (2006)
Article DOI: 10.1016/j.bmc.2006.09.026
BindingDB Entry DOI: 10.7270/Q2JS9Q2H |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10692

(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... |
J Med Chem 48: 4444-56 (2005)
Article DOI: 10.1021/jm049515h
BindingDB Entry DOI: 10.7270/Q2R78CF8 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10692

(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114606
BindingDB Entry DOI: 10.7270/Q2FJ2MW1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10692

(3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...)Show SMILES CNC(=O)Oc1cccc(CN(C)CCCOc2ccc3c(c2)oc2ccccc2c3=O)c1 Show InChI InChI=1S/C26H26N2O5/c1-27-26(30)32-20-8-5-7-18(15-20)17-28(2)13-6-14-31-19-11-12-22-24(16-19)33-23-10-4-3-9-21(23)25(22)29/h3-5,7-12,15-16H,6,13-14,17H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... |
J Med Chem 41: 3976-86 (1998)
Article DOI: 10.1021/jm9810046
BindingDB Entry DOI: 10.7270/Q2KH0KJ8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data