

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM131016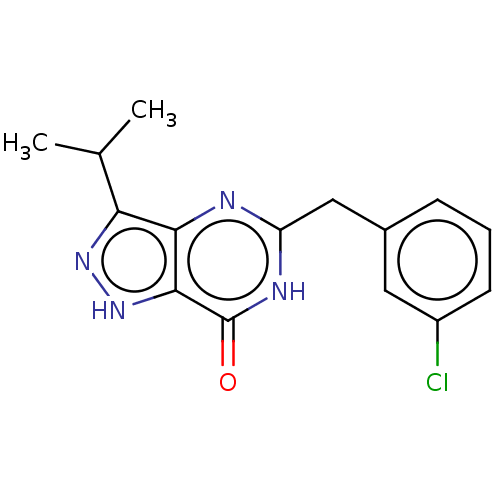 (US8829000, Referential Example 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 30 |
ASKA Pharmaceutical Co., Ltd. US Patent | Assay Description To 150 uL of buffer B (70 mmol/L Tris-HCl, pH7.5, 16.7 mmol/L MgCl2, 33.3 nmol/L [3H]-cGMP) solution containing [3H]-cGMP (specific activity=244.2 GB... | US Patent US8829000 (2014) BindingDB Entry DOI: 10.7270/Q2K072ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM131016 (US8829000, Referential Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
ASKA Pharmaceutical Co., Ltd. US Patent | Assay Description By a method similar to the measurement of PDE9-inhibiting activity, PDE5-inhibiting activity of each of the test compounds was measured, percent inhi... | US Patent US8829000 (2014) BindingDB Entry DOI: 10.7270/Q2K072ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||