

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142622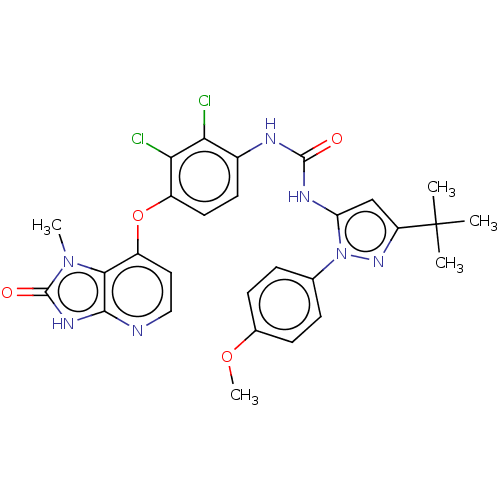 (US8933228, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM142622 (US8933228, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM142622 (US8933228, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 12 (Homo sapiens (Human)) | BDBM142622 (US8933228, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||