 Found 13 hits of ic50 for monomerid = 162872
Found 13 hits of ic50 for monomerid = 162872 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ATP-sensitive inward rectifier potassium channel 1
(Homo sapiens (Human)) | BDBM162872
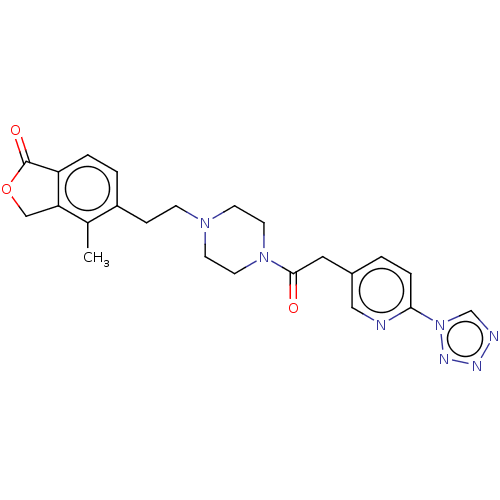
(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ROMK expressed in CHO cells by whole-cell voltage clamp method |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |
ATP-sensitive inward rectifier potassium channel 1
(Rattus norvegicus (Rat)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Block of Kir1.1 (ROMKI) currents was examined by whole cell voltage clamp (Hamill et. al. Pfluegers Archives 391:85-100 (1981)) using the IonWorks Qu... |
US Patent US9056859 (2015)
BindingDB Entry DOI: 10.7270/Q20P0XS0 |
More data for this
Ligand-Target Pair | |
ATP-sensitive inward rectifier potassium channel 1
(Rattus norvegicus (Rat)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat ROMK expressed in HEK293 cells after 30 mins by [86Rb+] flux functional assay |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |
ATP-sensitive inward rectifier potassium channel 1
(Rattus norvegicus (Rat)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat ROMK assessed as thallium flux after 30 mins in presence of ouabain by cell based FLIPR assay |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by electrophysiology analysis |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Homo sapiens (Human)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.2 (unknown origin) |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |
Voltage-dependent P/Q-type calcium channel subunit alpha-1A
(Homo sapiens (Human)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Cav2.1 (unknown origin) |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |
Inward rectifier potassium channel 2
(Homo sapiens (Human)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kir2.1 (unknown origin) |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |
Inward rectifier potassium channel 4
(Homo sapiens (Human)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kir2.3 (unknown origin) |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM162872

(US9056859, 72)Show SMILES Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 Show InChI InChI=1S/C23H25N7O3/c1-16-18(3-4-19-20(16)14-33-23(19)32)6-7-28-8-10-29(11-9-28)22(31)12-17-2-5-21(24-13-17)30-15-25-26-27-30/h2-5,13,15H,6-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 26: 2339-43 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.035
BindingDB Entry DOI: 10.7270/Q2668G3Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data