

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM163628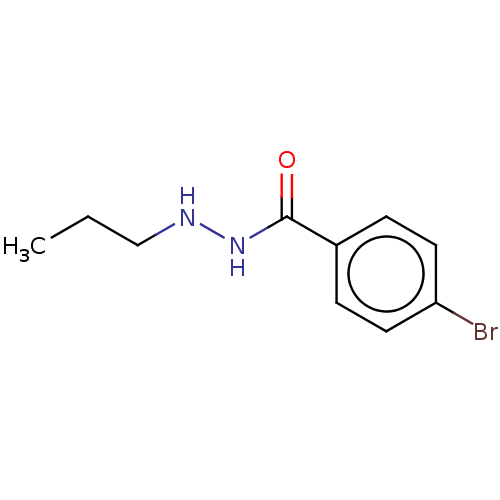 (SR-3212 | US10807944, Compound RLS2-137 | US117319...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2445RM9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM163628 (SR-3212 | US10807944, Compound RLS2-137 | US117319...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Research Foundation, Inc.; The Scripps Research Institute US Patent | Assay Description These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic... | US Patent US10807944 (2020) BindingDB Entry DOI: 10.7270/Q2M048J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3/Nuclear receptor corepressor 2 [395-498] (Homo sapiens (Human)) | BDBM163628 (SR-3212 | US10807944, Compound RLS2-137 | US117319...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine; Northwest Agriculture and Forestry University | Assay Description Purified HDAC1, HDAC2, and HDAC3 (in complex with the deacetylase activation domain of the human NCOR2 (amino acids 395¿498)) were obtained from BPS ... | Chem Biol 22: 273-84 (2015) Article DOI: 10.1016/j.chembiol.2014.12.015 BindingDB Entry DOI: 10.7270/Q2TX3D48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1/Nuclear receptor corepressor 2 [395-498] (Homo sapiens (Human)) | BDBM163628 (SR-3212 | US10807944, Compound RLS2-137 | US117319...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine; Northwest Agriculture and Forestry University | Assay Description Purified HDAC1, HDAC2, and HDAC3 (in complex with the deacetylase activation domain of the human NCOR2 (amino acids 395¿498)) were obtained from BPS ... | Chem Biol 22: 273-84 (2015) Article DOI: 10.1016/j.chembiol.2014.12.015 BindingDB Entry DOI: 10.7270/Q2TX3D48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM163628 (SR-3212 | US10807944, Compound RLS2-137 | US117319...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Research Foundation, Inc.; The Scripps Research Institute US Patent | Assay Description These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic... | US Patent US10807944 (2020) BindingDB Entry DOI: 10.7270/Q2M048J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM163628 (SR-3212 | US10807944, Compound RLS2-137 | US117319...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2445RM9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2/Nuclear receptor corepressor 2 [395-498] (Homo sapiens (Human)) | BDBM163628 (SR-3212 | US10807944, Compound RLS2-137 | US117319...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine; Northwest Agriculture and Forestry University | Assay Description Purified HDAC1, HDAC2, and HDAC3 (in complex with the deacetylase activation domain of the human NCOR2 (amino acids 395¿498)) were obtained from BPS ... | Chem Biol 22: 273-84 (2015) Article DOI: 10.1016/j.chembiol.2014.12.015 BindingDB Entry DOI: 10.7270/Q2TX3D48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM163628 (SR-3212 | US10807944, Compound RLS2-137 | US117319...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 3.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2445RM9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM163628 (SR-3212 | US10807944, Compound RLS2-137 | US117319...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Research Foundation, Inc.; The Scripps Research Institute US Patent | Assay Description These SAR data indicate that a tripartite structure of this scaffold with a central —C(O)—NH—NH— unit flanked by a phenyl group and a short aliphatic... | US Patent US10807944 (2020) BindingDB Entry DOI: 10.7270/Q2M048J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM163628 (SR-3212 | US10807944, Compound RLS2-137 | US117319...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine; Northwest Agriculture and Forestry University | Assay Description Purified HDAC1, HDAC2, and HDAC3 (in complex with the deacetylase activation domain of the human NCOR2 (amino acids 395¿498)) were obtained from BPS ... | Chem Biol 22: 273-84 (2015) Article DOI: 10.1016/j.chembiol.2014.12.015 BindingDB Entry DOI: 10.7270/Q2TX3D48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||