

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM16787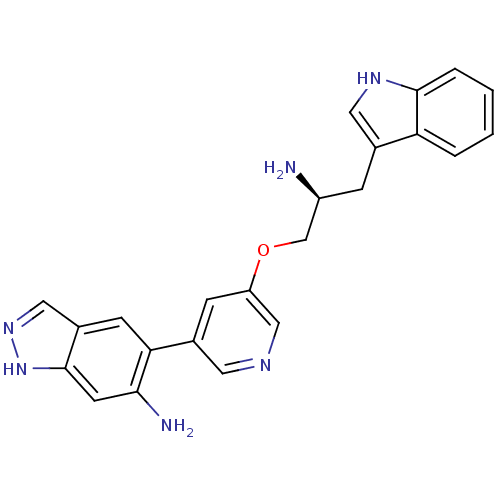 (5-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of Akt | Eur J Med Chem 44: 4090-7 (2009) Article DOI: 10.1016/j.ejmech.2009.04.050 BindingDB Entry DOI: 10.7270/Q2GT5N74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM16787 (5-{5-[(2S)-2-amino-3-(1H-indol-3-yl)propoxy]pyridi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem 15: 2441-52 (2007) Article DOI: 10.1016/j.bmc.2007.01.010 BindingDB Entry DOI: 10.7270/Q26Q1VGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||