

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172138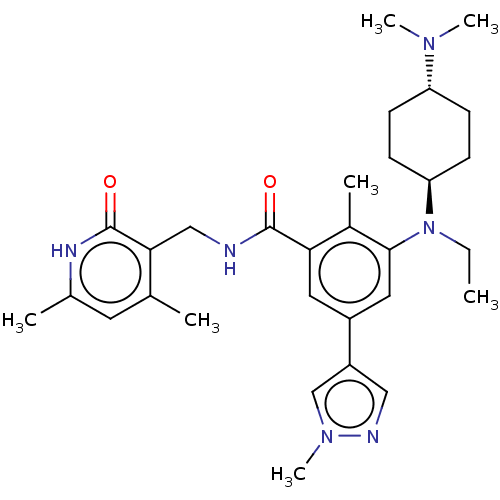 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat... | US Patent US9175331 (2015) BindingDB Entry DOI: 10.7270/Q2ZW1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [A677G] (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat... | US Patent US9175331 (2015) BindingDB Entry DOI: 10.7270/Q2ZW1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [A687V] (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat... | US Patent US9175331 (2015) BindingDB Entry DOI: 10.7270/Q2ZW1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat... | US Patent US9175331 (2015) BindingDB Entry DOI: 10.7270/Q2ZW1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.53 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM172138 (EPZ007428 | US10155002, Compound 145 | US11052093,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SB48WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||