

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM21625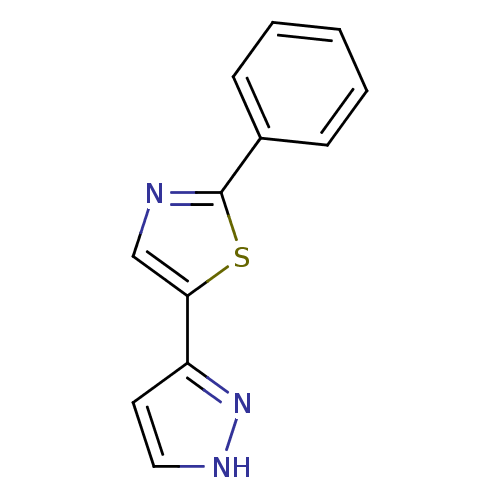 (2-phenyl-5-(1H-pyrazol-3-yl)-1,3-thiazole | 2-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.2 | 22 |
AstraZeneca | Assay Description The PGDS glutathione-S-transferase (GST) activity was measured by using MonoChloroBimane (MCB) as a chromogenic substrate. The assay was run at 384-w... | J Med Chem 51: 2178-86 (2008) Article DOI: 10.1021/jm701509k BindingDB Entry DOI: 10.7270/Q2Z036FR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM21625 (2-phenyl-5-(1H-pyrazol-3-yl)-1,3-thiazole | 2-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition human HPGDS expressed in Escherichia coli assessed as reduction in GST enzymatic activity using MCBL and glutathione incubated for 30 mins... | Bioorg Med Chem Lett 25: 2496-500 (2015) Article DOI: 10.1016/j.bmcl.2015.04.065 BindingDB Entry DOI: 10.7270/Q2J9683M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM21625 (2-phenyl-5-(1H-pyrazol-3-yl)-1,3-thiazole | 2-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM21625 (2-phenyl-5-(1H-pyrazol-3-yl)-1,3-thiazole | 2-phen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >6.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type human COMT expressed in Escherichia coli BL21 using 4-nitrocatechol-Alexa488 as substrate in presence of cofactor... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PK0KS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||