

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22873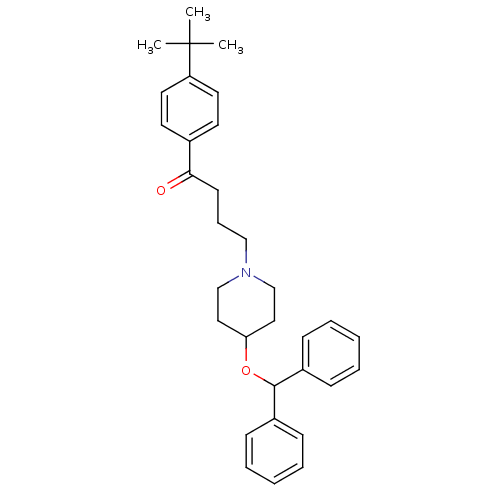 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound is evaluated for in vitro receptor binding affinity against H1 receptor | J Med Chem 38: 4026-32 (1995) BindingDB Entry DOI: 10.7270/Q2MK6DJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound is evaluated for in vitro receptor binding affinity against 5-hydroxytryptamine 1A receptor | J Med Chem 38: 4026-32 (1995) BindingDB Entry DOI: 10.7270/Q2MK6DJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound is evaluated for in vitro receptor binding affinity against Dopamine receptor D2 | J Med Chem 38: 4026-32 (1995) BindingDB Entry DOI: 10.7270/Q2MK6DJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
TCG Lifesciences Ltd. Curated by ChEMBL | Assay Description Inhibition of human ERG | Eur J Med Chem 46: 618-30 (2011) Article DOI: 10.1016/j.ejmech.2010.11.042 BindingDB Entry DOI: 10.7270/Q2WQ052W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea | Assay Description Ten-point DRCs were generated for each drug. Vero cells were seeded at 1.2 × 104 cells per well in DMEM, supplemented with 2% FBS and 1× ... | Antimicrob Agents Chemother 64: (2020) Article DOI: 10.1128/AAC.00819-20 BindingDB Entry DOI: 10.7270/Q22N54QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound is evaluated for in vitro receptor binding affinity against Muscarinic acetylcholine receptor M2 | J Med Chem 38: 4026-32 (1995) BindingDB Entry DOI: 10.7270/Q2MK6DJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound is evaluated for in vitro receptor binding affinity against Muscarinic acetylcholine receptor M1 | J Med Chem 38: 4026-32 (1995) BindingDB Entry DOI: 10.7270/Q2MK6DJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Inhibition of human ABCB1-mediated rhodamine 123 efflux in mouse L5178 cells expressing human MDR1 after 20 mins by FACS analysis | J Med Chem 54: 1740-51 (2011) Article DOI: 10.1021/jm101421d BindingDB Entry DOI: 10.7270/Q2C53M5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Reverse proteomics research institute Curated by ChEMBL | Assay Description Inhibitory concentration against potassium channel HERG | Bioorg Med Chem Lett 15: 2886-90 (2005) Article DOI: 10.1016/j.bmcl.2005.03.080 BindingDB Entry DOI: 10.7270/Q29S1S7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 15-lipoxygenase (Pseudomonas aeruginosa) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
University of California, Santa Cruz | Assay Description Briefly, 3 uL of enzyme (approximately 20 nM LoxA, final concentration) or buffer (no-enzyme control) was dispensed into 1536-well Greiner black clea... | Biochemistry 55: 3329-40 (2016) Article DOI: 10.1021/acs.biochem.6b00338 BindingDB Entry DOI: 10.7270/Q21N7ZXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 4 (Homo sapiens (Human)) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 3 (Homo sapiens (Human)) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-binding cassette sub-family C member 2 (Homo sapiens (Human)) | BDBM22873 (1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) Article DOI: 10.1093/toxsci/kft176 BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||