

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM29612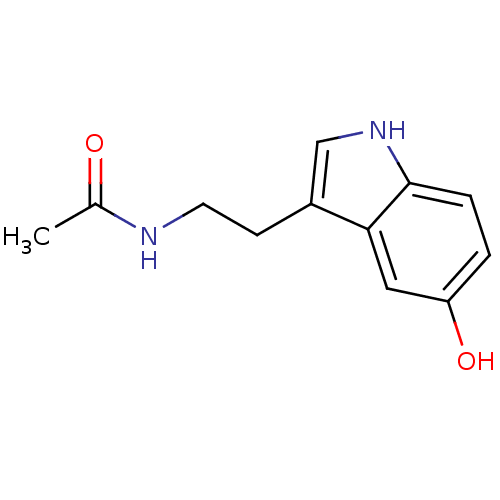 (CHEMBL33103 | CVD-0001578 | JOH-MSK-a63bdd1d-4 | N...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gr�nenthal GmbH Curated by ChEMBL | Assay Description Inhibition of human sepiapterin reductase using L-sepiapterin as substrate preincubated for 15 mins followed by substrate addition | J Med Chem 62: 6391-6397 (2019) Article DOI: 10.1021/acs.jmedchem.9b00218 BindingDB Entry DOI: 10.7270/Q2JW8J6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM29612 (CHEMBL33103 | CVD-0001578 | JOH-MSK-a63bdd1d-4 | N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
University of Illinois at Chicago | Assay Description The activity of recombinant human QR2 under steady-state conditions was evaluated on a MolecularDevices SpectraMax Plus 384 UV-visible spectrophotome... | Biochem J 413: 81-91 (2008) Article DOI: 10.1042/BJ20071373 BindingDB Entry DOI: 10.7270/Q2Z036G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Palmitoleoyl-protein carboxylesterase NOTUM (Homo sapiens (Human)) | BDBM29612 (CHEMBL33103 | CVD-0001578 | JOH-MSK-a63bdd1d-4 | N...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01974 BindingDB Entry DOI: 10.7270/Q2NP28F9 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM29612 (CHEMBL33103 | CVD-0001578 | JOH-MSK-a63bdd1d-4 | N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
Israel Institution of Biological Research | Assay Description The assay was performed according to the published procedure. Briefly, compounds were seeded into assay-ready plates (Greiner 384PP, cat# 781280) usi... | bioRxiv 2021: (2021) BindingDB Entry DOI: 10.7270/Q2MS3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM29612 (CHEMBL33103 | CVD-0001578 | JOH-MSK-a63bdd1d-4 | N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 9.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
Israel Institution of Biological Research | Assay Description Compounds were seeded into assay-ready plates (Greiner 384 low volume, cat 784900) using an Echo 555 acoustic dispenser, and DMSO was back-filled for... | bioRxiv 2021: (2021) BindingDB Entry DOI: 10.7270/Q2MS3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM29612 (CHEMBL33103 | CVD-0001578 | JOH-MSK-a63bdd1d-4 | N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 9.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM29612 (CHEMBL33103 | CVD-0001578 | JOH-MSK-a63bdd1d-4 | N...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology Curated by ChEMBL | Assay Description Inhibition of catecholase activity of tyrosinase in mouse B16 cells assessed as dopachrome formation | Bioorg Med Chem Lett 19: 4178-82 (2009) Article DOI: 10.1016/j.bmcl.2009.05.115 BindingDB Entry DOI: 10.7270/Q2BR8S70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||