

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM328998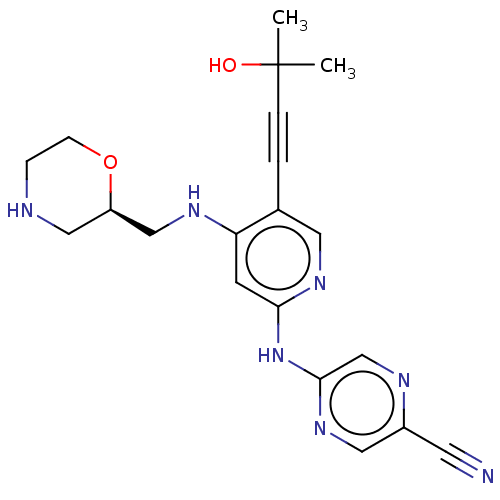 (1137478-52-4 | US11787792, Compound Y-158 | US9663...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CHK1 (unknown origin) using 5-FAM-KKKVSRSGLYRSPSMPENLNRPR-COOH peptide as substrate incubated for 1 hr in presence of ATP by caliper mi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01938 BindingDB Entry DOI: 10.7270/Q2MG7T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM328998 (1137478-52-4 | US11787792, Compound Y-158 | US9663...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM328998 (1137478-52-4 | US11787792, Compound Y-158 | US9663...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Limited US Patent | Assay Description CHK1 kinase activity was measured in a microfluidic assay that monitors the separation of a phosphorylated product from its substrate. The assay was ... | US Patent US9663503 (2017) BindingDB Entry DOI: 10.7270/Q2T43W7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM328998 (1137478-52-4 | US11787792, Compound Y-158 | US9663...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CHK1 in human HT-29 cells assessed as abrogation of etoposide-induced G2 checkpoint arrest by ELISA | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01938 BindingDB Entry DOI: 10.7270/Q2MG7T5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||