

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM339837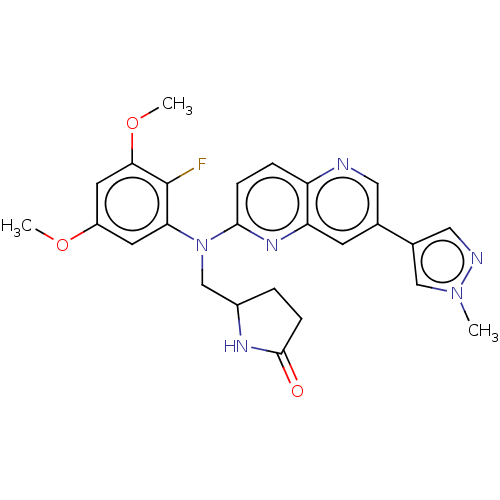 (US9757364, Compound 40 | US9757364, Compound 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LTD US Patent | Assay Description In a final reaction volume of 30 μL, FGFR1 (h) (25 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Trit... | US Patent US9757364 (2017) BindingDB Entry DOI: 10.7270/Q2SB47VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM339837 (US9757364, Compound 40 | US9757364, Compound 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LTD US Patent | Assay Description In a final reaction volume of 30 μL, FGFR1 (h) (25 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Trit... | US Patent US9757364 (2017) BindingDB Entry DOI: 10.7270/Q2SB47VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM339837 (US9757364, Compound 40 | US9757364, Compound 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LTD US Patent | Assay Description In a final reaction volume of 30 μL, FGFR3 (h) (40 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Trit... | US Patent US9757364 (2017) BindingDB Entry DOI: 10.7270/Q2SB47VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM339837 (US9757364, Compound 40 | US9757364, Compound 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LTD US Patent | Assay Description In a final reaction volume of 30 μL, FGFR3 (h) (40 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Trit... | US Patent US9757364 (2017) BindingDB Entry DOI: 10.7270/Q2SB47VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM339837 (US9757364, Compound 40 | US9757364, Compound 43) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LTD US Patent | Assay Description In a final reaction volume of 30 μL, FGFR4 (h) (60 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Trit... | US Patent US9757364 (2017) BindingDB Entry DOI: 10.7270/Q2SB47VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM339837 (US9757364, Compound 40 | US9757364, Compound 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LTD US Patent | Assay Description In a final reaction volume of 30 μL, FGFR2 (h) (150 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Tri... | US Patent US9757364 (2017) BindingDB Entry DOI: 10.7270/Q2SB47VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM339837 (US9757364, Compound 40 | US9757364, Compound 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.14 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LTD US Patent | Assay Description In a final reaction volume of 30 μL, FGFR2 (h) (150 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Tri... | US Patent US9757364 (2017) BindingDB Entry DOI: 10.7270/Q2SB47VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 (Homo sapiens (Human)) | BDBM339837 (US9757364, Compound 40 | US9757364, Compound 43) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LTD US Patent | Assay Description In a final reaction volume of 30 μL, FGFR4 (h) (60 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Trit... | US Patent US9757364 (2017) BindingDB Entry DOI: 10.7270/Q2SB47VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM339837 (US9757364, Compound 40 | US9757364, Compound 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LTD US Patent | Assay Description In a final reaction volume of 30 μL, KDR (h) (150 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Trito... | US Patent US9757364 (2017) BindingDB Entry DOI: 10.7270/Q2SB47VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM339837 (US9757364, Compound 40 | US9757364, Compound 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21.4 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LTD US Patent | Assay Description In a final reaction volume of 30 μL, KDR (h) (150 ng/ml) was incubated with 50 mM HEPES pH 7.5, 6 mM MnCl2, 1 mM DTT, 0.1 mM Na3VO4, 0.01% Trito... | US Patent US9757364 (2017) BindingDB Entry DOI: 10.7270/Q2SB47VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||