 Found 9 hits of ic50 for monomerid = 4703
Found 9 hits of ic50 for monomerid = 4703 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM4703
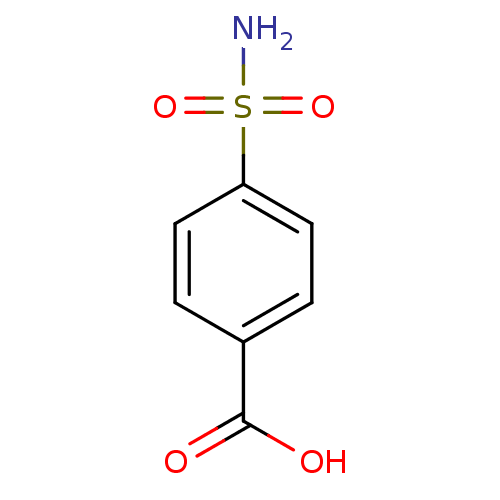
(4-carboxybenzenesulfonamide 1 | 4-sulfamoylbenzoic...)Show InChI InChI=1S/C7H7NO4S/c8-13(11,12)6-3-1-5(2-4-6)7(9)10/h1-4H,(H,9,10)(H2,8,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cystolic isozyme II of Carbonic anhydrase |
Bioorg Med Chem Lett 14: 5703-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.085
BindingDB Entry DOI: 10.7270/Q23N22VW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 5A, mitochondrial
(Homo sapiens (Human)) | BDBM4703

(4-carboxybenzenesulfonamide 1 | 4-sulfamoylbenzoic...)Show InChI InChI=1S/C7H7NO4S/c8-13(11,12)6-3-1-5(2-4-6)7(9)10/h1-4H,(H,9,10)(H2,8,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cystolic isozyme V of Carbonic anhydrase |
Bioorg Med Chem Lett 14: 5703-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.085
BindingDB Entry DOI: 10.7270/Q23N22VW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM4703

(4-carboxybenzenesulfonamide 1 | 4-sulfamoylbenzoic...)Show InChI InChI=1S/C7H7NO4S/c8-13(11,12)6-3-1-5(2-4-6)7(9)10/h1-4H,(H,9,10)(H2,8,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM4703

(4-carboxybenzenesulfonamide 1 | 4-sulfamoylbenzoic...)Show InChI InChI=1S/C7H7NO4S/c8-13(11,12)6-3-1-5(2-4-6)7(9)10/h1-4H,(H,9,10)(H2,8,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA2 using 4-nitrophenylacetate as substrate by esterase assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128420
BindingDB Entry DOI: 10.7270/Q2VX0MC2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM4703

(4-carboxybenzenesulfonamide 1 | 4-sulfamoylbenzoic...)Show InChI InChI=1S/C7H7NO4S/c8-13(11,12)6-3-1-5(2-4-6)7(9)10/h1-4H,(H,9,10)(H2,8,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA9 using 4-nitrophenylacetate as substrate by esterase assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128420
BindingDB Entry DOI: 10.7270/Q2VX0MC2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM4703

(4-carboxybenzenesulfonamide 1 | 4-sulfamoylbenzoic...)Show InChI InChI=1S/C7H7NO4S/c8-13(11,12)6-3-1-5(2-4-6)7(9)10/h1-4H,(H,9,10)(H2,8,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM4703

(4-carboxybenzenesulfonamide 1 | 4-sulfamoylbenzoic...)Show InChI InChI=1S/C7H7NO4S/c8-13(11,12)6-3-1-5(2-4-6)7(9)10/h1-4H,(H,9,10)(H2,8,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA1 using 4-nitrophenylacetate as substrate by esterase assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128420
BindingDB Entry DOI: 10.7270/Q2VX0MC2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM4703

(4-carboxybenzenesulfonamide 1 | 4-sulfamoylbenzoic...)Show InChI InChI=1S/C7H7NO4S/c8-13(11,12)6-3-1-5(2-4-6)7(9)10/h1-4H,(H,9,10)(H2,8,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (N9)) | BDBM4703

(4-carboxybenzenesulfonamide 1 | 4-sulfamoylbenzoic...)Show InChI InChI=1S/C7H7NO4S/c8-13(11,12)6-3-1-5(2-4-6)7(9)10/h1-4H,(H,9,10)(H2,8,11,12) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+6 | n/a | n/a | n/a | n/a | 6.5 | 37 |
BioCryst Pharmaceuticals, Inc.
| Assay Description
A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... |
J Med Chem 40: 4030-52 (1997)
Article DOI: 10.1021/jm970479e
BindingDB Entry DOI: 10.7270/Q2X63K41 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data