

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphoethanolamine/phosphocholine phosphatase (Homo sapiens (Human)) | BDBM47032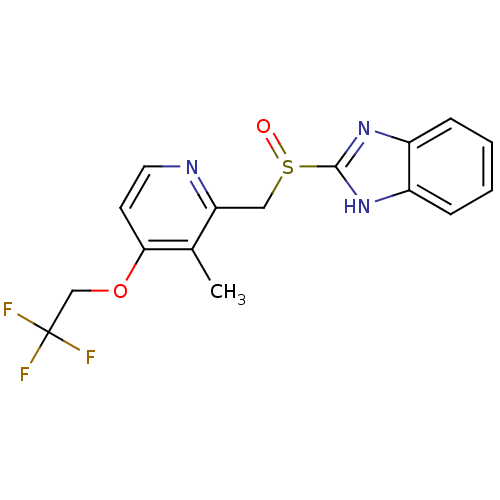 (2-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl...) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 434 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q27S7M52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD(+) hydrolase SARM1 (Homo sapiens) | US11903935, Compound IA-2 (2-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD(+) hydrolase SARM1 (Homo sapiens) | US11903935, Compound IA-2 (2-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Mus musculus) | BDBM47032 (2-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of mouse Ido2 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom... | Bioorg Med Chem Lett 22: 7641-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.010 BindingDB Entry DOI: 10.7270/Q20G3M92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM47032 (2-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Clinical Pharmacology Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Digoxin transepithelial transport (basal to apical) (Digoxin: 5 uM) in Caco-2 cells | Naunyn Schmiedebergs Arch Pharmacol 364: 551-7 (2001) BindingDB Entry DOI: 10.7270/Q2MK6F4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM47032 (2-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of mouse Ido1 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom... | Bioorg Med Chem Lett 22: 7641-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.010 BindingDB Entry DOI: 10.7270/Q20G3M92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||