

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003356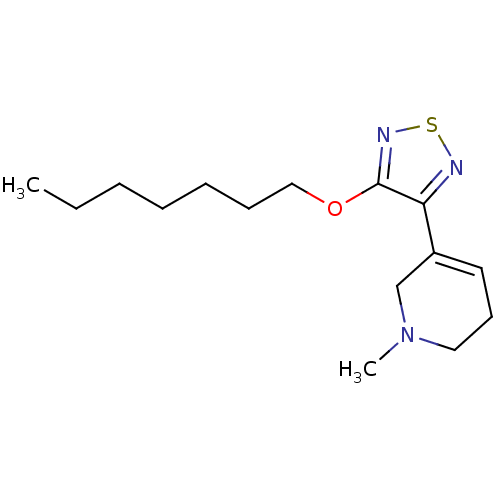 (5-(4-Heptyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-oxotremorine-M (Oxo-M) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003356 (5-(4-Heptyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against rat hippocampus M1 receptor using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50003356 (5-(4-Heptyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50003356 (5-(4-Heptyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Efficacy at muscarinic acetylcholine receptor M1 measured by the ability to inhibit the electrically stimulated twitch of the rabbit vas deferens | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003356 (5-(4-Heptyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk CNS Division Curated by ChEMBL | Assay Description In vitro binding affinity against muscarinic acetylcholine receptor M1 from rat hippocampus, using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 2274-83 (1992) BindingDB Entry DOI: 10.7270/Q22J69TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003356 (5-(4-Heptyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-pirenzepine (Pz) from rat hippocampus muscarinic acetylcholine receptor M1 | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||