

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004152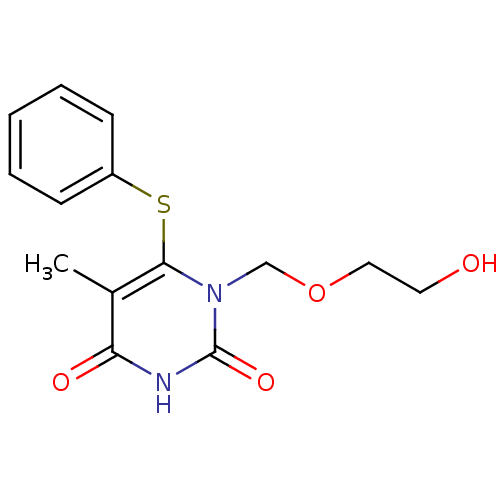 ((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells | Bioorg Med Chem Lett 14: 3173-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.008 BindingDB Entry DOI: 10.7270/Q2862HNN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004152 ((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004152 ((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 reverse transcriptase | J Med Chem 39: 1589-600 (1996) Article DOI: 10.1021/jm960056x BindingDB Entry DOI: 10.7270/Q23X85QC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004152 ((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description Inhibitory concentration required to inhibit the HIV-1 reverse transcriptase activity | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004152 ((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase from peripheral blood mononuclear cells. | J Med Chem 34: 3305-9 (1991) BindingDB Entry DOI: 10.7270/Q2DR2TFF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004152 ((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa University Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 reverse transcriptase activity using poly(rC)-oligo(dG) template primer | J Med Chem 32: 2507-9 (1989) BindingDB Entry DOI: 10.7270/Q2HH6J27 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004152 ((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | >4.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibitory concentration against SO561945 (HIV 1 mutant RT) viral viral infection of MT-4 cells | Bioorg Med Chem Lett 14: 3173-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.008 BindingDB Entry DOI: 10.7270/Q2862HNN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004152 ((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa University Curated by ChEMBL | Assay Description Inhibitory effect of the compound on HIV-1 reverse transcriptase activity using poly(rA)-oligo(dT) template primer | J Med Chem 32: 2507-9 (1989) BindingDB Entry DOI: 10.7270/Q2HH6J27 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||