 Found 9 hits of ic50 for monomerid = 50029799
Found 9 hits of ic50 for monomerid = 50029799 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50029799
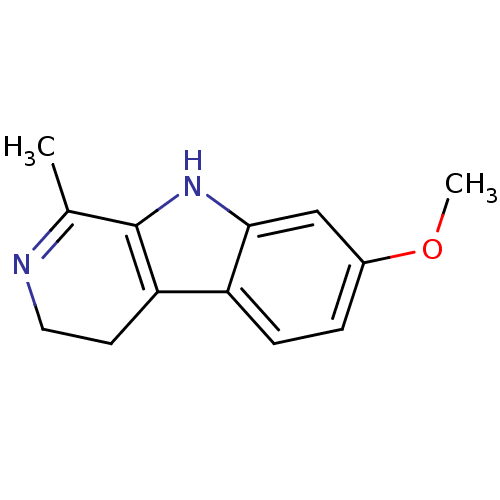
(7-Methoxy-1-methyl-2,9-dihydro-1H-beta-carboline |...)Show InChI InChI=1S/C13H14N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-4,7,15H,5-6H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Rattus norvegicus (rat)) | BDBM50029799

(7-Methoxy-1-methyl-2,9-dihydro-1H-beta-carboline |...)Show InChI InChI=1S/C13H14N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-4,7,15H,5-6H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris 7
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat brain Monoamine oxidase A |
J Med Chem 38: 4786-92 (1996)
BindingDB Entry DOI: 10.7270/Q2T72GFV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50029799

(7-Methoxy-1-methyl-2,9-dihydro-1H-beta-carboline |...)Show InChI InChI=1S/C13H14N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-4,7,15H,5-6H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI-TN-5B1-4 insect cells using p-tyramine as substrate preincubated for 15 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112911
BindingDB Entry DOI: 10.7270/Q2RR22XQ |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50029799

(7-Methoxy-1-methyl-2,9-dihydro-1H-beta-carboline |...)Show InChI InChI=1S/C13H14N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-4,7,15H,5-6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50029799

(7-Methoxy-1-methyl-2,9-dihydro-1H-beta-carboline |...)Show InChI InChI=1S/C13H14N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-4,7,15H,5-6H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylcholine iodide as substrate measured every 5 sec for 2 mins by Ellman's method |
Bioorg Med Chem Lett 22: 2885-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.057
BindingDB Entry DOI: 10.7270/Q2251K5M |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50029799

(7-Methoxy-1-methyl-2,9-dihydro-1H-beta-carboline |...)Show InChI InChI=1S/C13H14N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-4,7,15H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Naresuan University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's method |
Bioorg Med Chem Lett 22: 2885-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.057
BindingDB Entry DOI: 10.7270/Q2251K5M |
More data for this
Ligand-Target Pair | |
Snake venom metalloproteinase BaP1
(Bothrops asper) | BDBM50029799

(7-Methoxy-1-methyl-2,9-dihydro-1H-beta-carboline |...)Show InChI InChI=1S/C13H14N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-4,7,15H,5-6H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of snake venom BaP1 using Abz-Ala-Gly-Leu-Ala-Nba as substrate incubated for 30 mins prior to substrate addition by fluorescence spectroph... |
ACS Med Chem Lett 3: 540-543 (2012)
Article DOI: 10.1021/ml300068r
BindingDB Entry DOI: 10.7270/Q2542PVS |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50029799

(7-Methoxy-1-methyl-2,9-dihydro-1H-beta-carboline |...)Show InChI InChI=1S/C13H14N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-4,7,15H,5-6H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-B expressed in baculovirus infected BTI-TN-5B1-4 insect cells using p-tyramine as substrate preincubated for 15 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112911
BindingDB Entry DOI: 10.7270/Q2RR22XQ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Rattus norvegicus (rat)) | BDBM50029799

(7-Methoxy-1-methyl-2,9-dihydro-1H-beta-carboline |...)Show InChI InChI=1S/C13H14N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-4,7,15H,5-6H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris 7
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat brain Monoamine oxidase B |
J Med Chem 38: 4786-92 (1996)
BindingDB Entry DOI: 10.7270/Q2T72GFV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data