

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50042056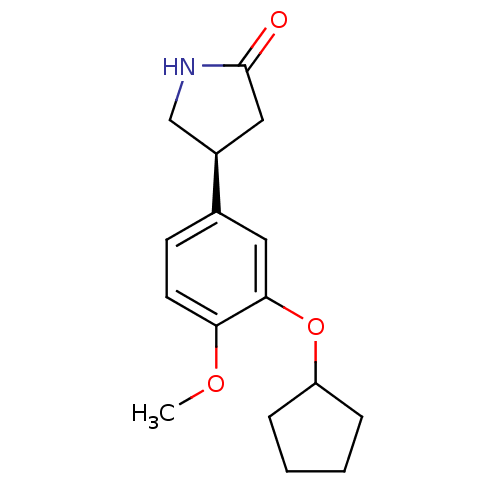 ((R)-4-(3-Cyclopentyloxy-4-methoxy-phenyl)-pyrrolid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50042056 ((R)-4-(3-Cyclopentyloxy-4-methoxy-phenyl)-pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of PDE4D2 catalytic domain (86 to 413 residues) (unknown origin) | Eur J Med Chem 114: 134-40 (2016) Article DOI: 10.1016/j.ejmech.2015.12.002 BindingDB Entry DOI: 10.7270/Q2765H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50042056 ((R)-4-(3-Cyclopentyloxy-4-methoxy-phenyl)-pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of PDE4D2 catalytic domain (86 to 413 residues) (unknown origin) using [3H]-cAMP as substrate after 15 mins by liquid scintillation counti... | Eur J Med Chem 114: 134-40 (2016) Article DOI: 10.1016/j.ejmech.2015.12.002 BindingDB Entry DOI: 10.7270/Q2765H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50042056 ((R)-4-(3-Cyclopentyloxy-4-methoxy-phenyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition activity against human monocyte derived PDE4 catalytic activity (LPDE4) | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50042056 ((R)-4-(3-Cyclopentyloxy-4-methoxy-phenyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of partially-purified PDE 4 from human monocytes | J Med Chem 36: 3274-7 (1993) Article DOI: 10.1021/jm00074a007 BindingDB Entry DOI: 10.7270/Q24M96RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50042056 ((R)-4-(3-Cyclopentyloxy-4-methoxy-phenyl)-pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||