

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50046267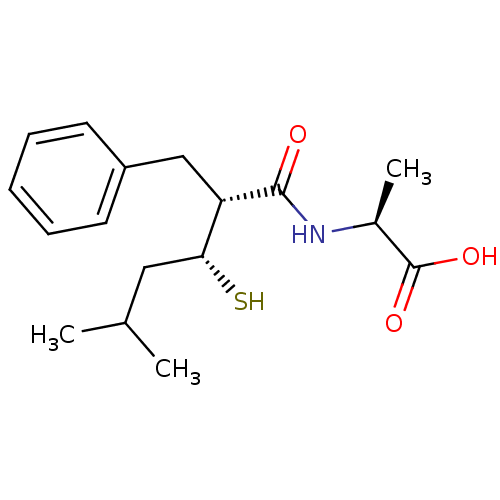 ((RRS) 2-(2-Benzyl-3-mercapto-5-methyl-hexanoylamin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated by Inhibiting 50% of Neutral endopeptidase enzyme (NEP) activity using 20 nM [3H]-D-Ala2-Leu-enkephalin as sub... | J Med Chem 36: 87-94 (1993) BindingDB Entry DOI: 10.7270/Q26972NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50046267 ((RRS) 2-(2-Benzyl-3-mercapto-5-methyl-hexanoylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated by inhibiting 50% of Angiotensin I Converting Enzyme activity using 50 microM N-Cbz-Phe-His-Leu as substrate | J Med Chem 36: 87-94 (1993) BindingDB Entry DOI: 10.7270/Q26972NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||