

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050636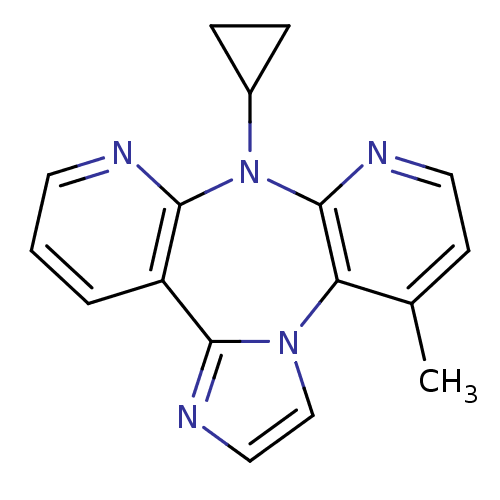 (8-Cyclopropyl-4-methyl-8H-1,3a,7,8,9-pentaaza-dibe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rN.dN template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050636 (8-Cyclopropyl-4-methyl-8H-1,3a,7,8,9-pentaaza-dibe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rA.dT template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050636 (8-Cyclopropyl-4-methyl-8H-1,3a,7,8,9-pentaaza-dibe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||