

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051117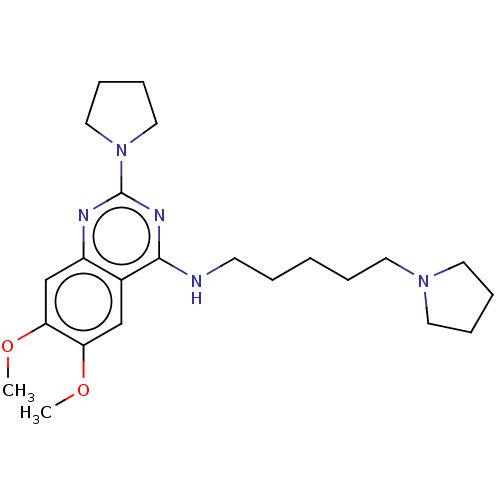 (CHEMBL3318285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of SETD8 (unknown origin) catalytic domain expressed in Escherichia coli using biotinylated H4 (1-24) peptide substrate after 1 hr by scin... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of methyltransferase activity of SETD8 (unknown origin) catalytic domain expressed in Escherichia coli assessed as decrease in methylation... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Displacement of FITC-labeled H4K20me (1-24) from SETD8 (unknown origin) catalytic domain expressed in Escherichia coli by fluorescence polarization a... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SUV39H2 (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of SUV39H2 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate ... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of SETD7 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate by... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of DOT1L (unknown origin) by filter-based assay | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of PRMT5 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate by... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of PRMT1 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate by... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase KMT5B (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of SUV420H1 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase KMT5C (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of SUV420H2 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate by... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of DNMT1 (unknown origin) assessed as using dsDNA substrate by radioactive assay | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of MLL1 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate by ... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT1 (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of GLP (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate by r... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETDB1 (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of SETDB1 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate b... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate by r... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM50051117 (CHEMBL3318285) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of PRMT3 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate by... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||