

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50055821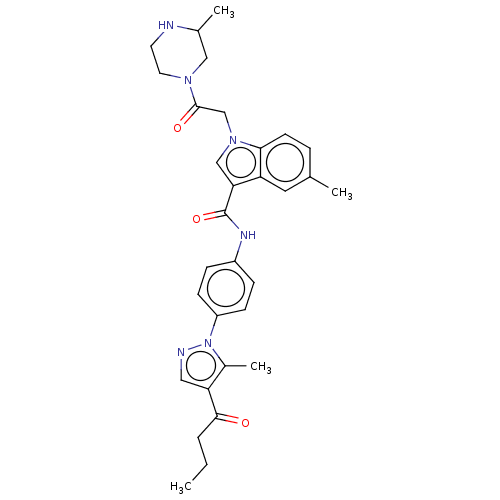 (CHEMBL3325665) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Rattus norvegicus) | BDBM50055821 (CHEMBL3325665) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||