 Found 5 hits of ic50 for monomerid = 50060582
Found 5 hits of ic50 for monomerid = 50060582 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50060582
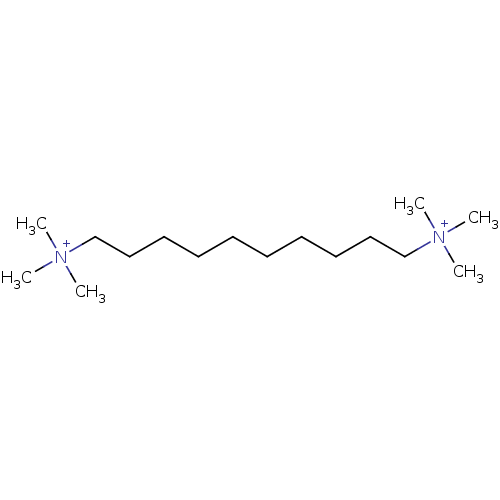
(1,10-Bis(trimethyl ammonium)decane dibromide | 1,1...)Show InChI InChI=1S/C16H38N2/c1-17(2,3)15-13-11-9-7-8-10-12-14-16-18(4,5)6/h7-16H2,1-6H3/q+2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of histone H3 receptor |
Bioorg Med Chem 16: 2968-73 (2008)
Article DOI: 10.1016/j.bmc.2007.12.048
BindingDB Entry DOI: 10.7270/Q27S7PMQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50060582

(1,10-Bis(trimethyl ammonium)decane dibromide | 1,1...)Show InChI InChI=1S/C16H38N2/c1-17(2,3)15-13-11-9-7-8-10-12-14-16-18(4,5)6/h7-16H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholine esterase (unknown origin) |
Bioorg Med Chem 22: 4474-89 (2014)
Article DOI: 10.1016/j.bmc.2014.04.019
BindingDB Entry DOI: 10.7270/Q29K4CW0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Torpedo marmorata (Marbled electric ray)) | BDBM50060582

(1,10-Bis(trimethyl ammonium)decane dibromide | 1,1...)Show InChI InChI=1S/C16H38N2/c1-17(2,3)15-13-11-9-7-8-10-12-14-16-18(4,5)6/h7-16H2,1-6H3/q+2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | 4.0 | 25 |
Université Louis Pasteur
| Assay Description
The inhibitory activity of the equilibrated mixtures was determined. The activity of AChE was monitored by following the linear change in absorbance... |
Chembiochem 2: 438-44 (2001)
Article DOI: 10.1002/1439-7633(20010601)2
BindingDB Entry DOI: 10.7270/Q2J964W8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50060582

(1,10-Bis(trimethyl ammonium)decane dibromide | 1,1...)Show InChI InChI=1S/C16H38N2/c1-17(2,3)15-13-11-9-7-8-10-12-14-16-18(4,5)6/h7-16H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Bioorg Med Chem 16: 2968-73 (2008)
Article DOI: 10.1016/j.bmc.2007.12.048
BindingDB Entry DOI: 10.7270/Q27S7PMQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phospholipase B
(Cryptococcus neoformans) | BDBM50060582

(1,10-Bis(trimethyl ammonium)decane dibromide | 1,1...)Show InChI InChI=1S/C16H38N2/c1-17(2,3)15-13-11-9-7-8-10-12-14-16-18(4,5)6/h7-16H2,1-6H3/q+2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of Cryptococccus neoformans H99 PLB1activity |
J Med Chem 49: 811-6 (2006)
Article DOI: 10.1021/jm0508843
BindingDB Entry DOI: 10.7270/Q2M61JT1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data