

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50061625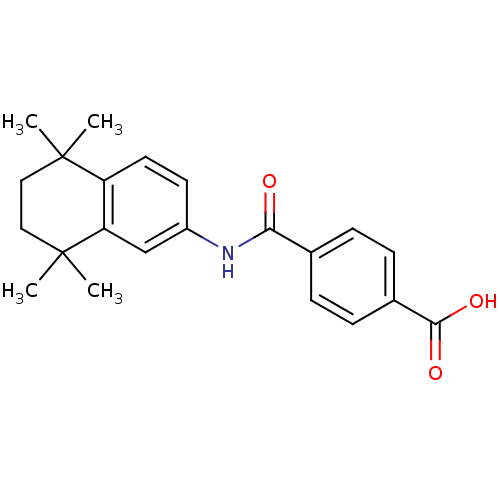 (4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) | J Med Chem 43: 409-19 (2000) BindingDB Entry DOI: 10.7270/Q28S4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50061625 (4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM50061625 (4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle | Assay Description Eighteen compounds were tested as potential inhibitors of CYP26A1 and CYP26B1. The formation of 9-cis-4-OH-RA metabolite was monitored and the percen... | J Med Chem 52: 1864-72 (2009) BindingDB Entry DOI: 10.7270/Q2FN18J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50061625 (4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Shandong University | Assay Description Briefly, the assay was performed in 96-well plates in 50 mm PBS, pH 7.2 as the assay buffer, at 37 °C. The APN solution was mixed with compounds at v... | Chem Biol Drug Des 88: 542-55 (2016) Article DOI: 10.1111/cbdd.12778 BindingDB Entry DOI: 10.7270/Q2HX1BHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50061625 (4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University | Assay Description Briefly, the HDAC8 enzyme was diluted 20 times. Then, 10 μL of HDAC8 solution was mixed with compounds at various concentrations (50 μL). T... | Chem Biol Drug Des 88: 542-55 (2016) Article DOI: 10.1111/cbdd.12778 BindingDB Entry DOI: 10.7270/Q2HX1BHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Yersinia pestis) | BDBM50061625 (4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawai'i at Hilo Curated by ChEMBL | Assay Description Inhibition of Yersinia pestis topoisomerase 1-mediated relaxation of supercoiled plasmid DNA by agarose gel electrophoresis | Medchemcomm 4: (2013) Article DOI: 10.1039/C3MD00238A BindingDB Entry DOI: 10.7270/Q23F4SM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||