

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kynurenine 3-monooxygenase (Rattus norvegicus) | BDBM50061916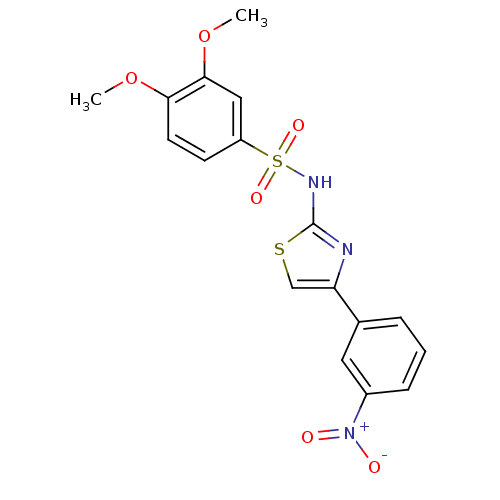 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management/CHDI Foundation Curated by ChEMBL | Assay Description Inhibition of Wistar rat KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS analy... | Drug Metab Dispos 40: 2297-306 (2012) Article DOI: 10.1124/dmd.112.046532 BindingDB Entry DOI: 10.7270/Q2WW7KDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Rattus norvegicus) | BDBM50061916 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of rat kidney Kynurenine 3-hydroxylase. | J Med Chem 40: 4378-85 (1998) Article DOI: 10.1021/jm970467t BindingDB Entry DOI: 10.7270/Q24B30DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50061916 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of Phosphodiesterase 2 from pig coronary artery | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50061916 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado College Curated by ChEMBL | Assay Description Inhibition of human KMO | J Med Chem 58: 8762-82 (2015) Article DOI: 10.1021/acs.jmedchem.5b00461 BindingDB Entry DOI: 10.7270/Q2C82C3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50061916 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Inhibition of KMO (unknown origin) | Bioorg Med Chem Lett 27: 1705-1708 (2017) Article DOI: 10.1016/j.bmcl.2017.02.080 BindingDB Entry DOI: 10.7270/Q2154K97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50061916 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management/CHDI Foundation Curated by ChEMBL | Assay Description Inhibition of C57BL/6J mouse KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS a... | Drug Metab Dispos 40: 2297-306 (2012) Article DOI: 10.1124/dmd.112.046532 BindingDB Entry DOI: 10.7270/Q2WW7KDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50061916 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management/CHDI Foundation Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS analysis | Drug Metab Dispos 40: 2297-306 (2012) Article DOI: 10.1124/dmd.112.046532 BindingDB Entry DOI: 10.7270/Q2WW7KDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Rattus norvegicus) | BDBM50061916 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management/CHDI Foundation Curated by ChEMBL | Assay Description Inhibition of Wistar rat KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS analy... | Drug Metab Dispos 40: 2297-306 (2012) Article DOI: 10.1124/dmd.112.046532 BindingDB Entry DOI: 10.7270/Q2WW7KDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Mus musculus) | BDBM50061916 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management/CHDI Foundation Curated by ChEMBL | Assay Description Inhibition of C57BL/6J mouse KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS a... | Drug Metab Dispos 40: 2297-306 (2012) Article DOI: 10.1124/dmd.112.046532 BindingDB Entry DOI: 10.7270/Q2WW7KDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Homo sapiens (Human)) | BDBM50061916 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management/CHDI Foundation Curated by ChEMBL | Assay Description Inhibition of human KMO assessed as 3-hydroxykynurenine formation preincubated for 5 mins using 100 uM kynurenine as substrate by LC-MS/MS analysis i... | Drug Metab Dispos 40: 2297-306 (2012) Article DOI: 10.1124/dmd.112.046532 BindingDB Entry DOI: 10.7270/Q2WW7KDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||