

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 1 (Mus musculus) | BDBM50065290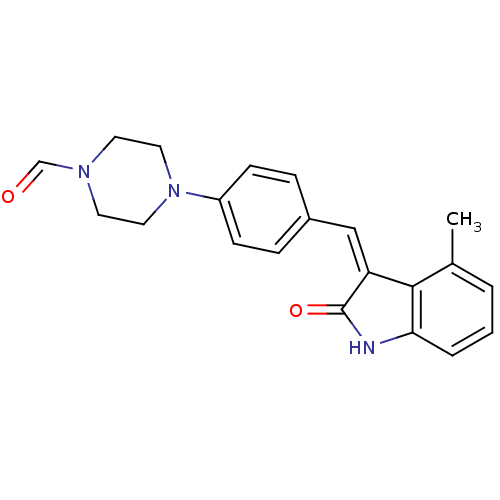 ((Z)-4-(4-((4-methyl-2-oxoindolin-3-ylidene)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc. Curated by ChEMBL | Assay Description Concentration required to achieve 50% inhibition of tyrosine phosphorylation on murine VEGF receptor (FLK-1 RTK). | J Med Chem 41: 2588-603 (1998) Article DOI: 10.1021/jm980123i BindingDB Entry DOI: 10.7270/Q2G73CTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Mus musculus) | BDBM50065290 ((Z)-4-(4-((4-methyl-2-oxoindolin-3-ylidene)methyl)...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) | J Med Chem 43: 3020-32 (2000) BindingDB Entry DOI: 10.7270/Q2HM59P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50065290 ((Z)-4-(4-((4-methyl-2-oxoindolin-3-ylidene)methyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milano-Bicocca Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged RET expressed in Sf9 insect cells | Bioorg Med Chem 18: 1482-96 (2010) Article DOI: 10.1016/j.bmc.2010.01.011 BindingDB Entry DOI: 10.7270/Q2B56KPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50065290 ((Z)-4-(4-((4-methyl-2-oxoindolin-3-ylidene)methyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc. Curated by ChEMBL | Assay Description Test concentration required to achieve 50% inhibition of tyrosine phosphorylation on human Platelet-derived growth factor receptor beta (PDGF RTK). | J Med Chem 41: 2588-603 (1998) Article DOI: 10.1021/jm980123i BindingDB Entry DOI: 10.7270/Q2G73CTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50065290 ((Z)-4-(4-((4-methyl-2-oxoindolin-3-ylidene)methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc. Curated by ChEMBL | Assay Description Concentration required to achieve 50% inhibition of tyrosine phosphorylation on human Insulin-like growth factor I receptor | J Med Chem 41: 2588-603 (1998) Article DOI: 10.1021/jm980123i BindingDB Entry DOI: 10.7270/Q2G73CTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50065290 ((Z)-4-(4-((4-methyl-2-oxoindolin-3-ylidene)methyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc. Curated by ChEMBL | Assay Description Concentration required to achieve 50% inhibition of tyrosine phosphorylation on human Epidermal growth factor receptor (EGF RTK). | J Med Chem 41: 2588-603 (1998) Article DOI: 10.1021/jm980123i BindingDB Entry DOI: 10.7270/Q2G73CTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50065290 ((Z)-4-(4-((4-methyl-2-oxoindolin-3-ylidene)methyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc. Curated by ChEMBL | Assay Description Concentration required to achieve 50% inhibition of tyrosine phosphorylation on human Her-2 receptor tyrosine kinase (HER-2 RTK) | J Med Chem 41: 2588-603 (1998) Article DOI: 10.1021/jm980123i BindingDB Entry DOI: 10.7270/Q2G73CTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||