

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 [A687V] (Homo sapiens (Human)) | BDBM50075051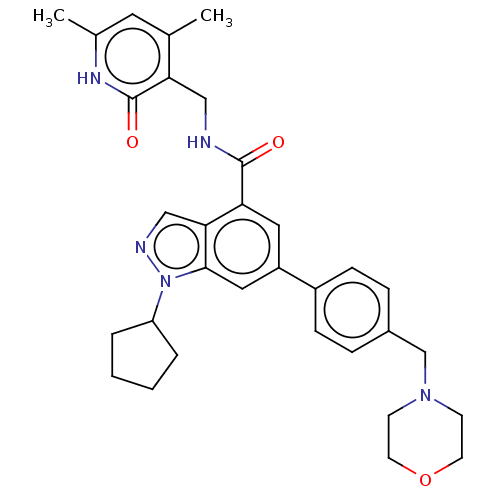 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat... | US Patent US9175331 (2015) BindingDB Entry DOI: 10.7270/Q2ZW1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [A677G] (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat... | US Patent US9175331 (2015) BindingDB Entry DOI: 10.7270/Q2ZW1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH1 (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH1 in PRC2 complex (unknown origin) using biotinylated peptide as substrate in presence of S-adenosylmethionine | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EZH2 in human PRC2 complex preincubated for 30 mins followed by histone h3 peptide (21 to 44 residues) and S-adenosylmethionine and mea... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01208 BindingDB Entry DOI: 10.7270/Q25X2DZR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114419 BindingDB Entry DOI: 10.7270/Q2PR80Z0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [Y641F] (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat... | US Patent US9175331 (2015) BindingDB Entry DOI: 10.7270/Q2ZW1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of wild-type EZH2 in human OCI-LY19 cells assessed as reduction of H3K27me3 level after 96 hrs by ELISA assay | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Test compounds were serially diluted 3-fold in DMSO in a 10 point-curve and 1 uL was spotted into a 384-well microplate in duplicate using a Platemat... | US Patent US9175331 (2015) BindingDB Entry DOI: 10.7270/Q2ZW1JQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <300 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description eneral Procedure for Wild-Type PRC2 Enzyme Assay on Oligonucleosome Substrate. The assays was performed in a buffer consisting of 20 mM bicine (pH=7.... | J Med Chem 51: 2350-3 (2008) BindingDB Entry DOI: 10.7270/Q28P62V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <300 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Recombinant PRC2 Enzymes. Human PRC2 enzymes were purified as 4-component enzyme complexes co-expressed in Spodoptera frugiperda (sf9) cells using a ... | US Patent US9637472 (2017) BindingDB Entry DOI: 10.7270/Q2X0693B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00047 BindingDB Entry DOI: 10.7270/Q27D3059 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human EZH2 using SAM and histone H3 (16 to 30) as substrate preincubated for 30 mins followed by substrate addition measured after 90 m... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||