 Found 11 hits of ic50 for monomerid = 50083351
Found 11 hits of ic50 for monomerid = 50083351 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50083351
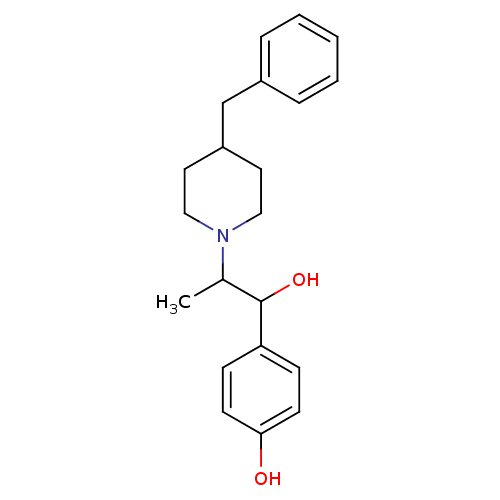
((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...)Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Messina
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ifenprodil from NMDA receptor GluN2B subunit in Wistar rat cerebral cortex |
Bioorg Med Chem 22: 1040-8 (2014)
Article DOI: 10.1016/j.bmc.2013.12.040
BindingDB Entry DOI: 10.7270/Q29888GS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50083351

((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...)Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Farmaceutiche ed Ambientali (CHIBIOFARAM) Universit£ degli Studi di Messina
Curated by ChEMBL
| Assay Description
Displacement of [3H]ifenprodil from Wistar rat cerebral cortex GluN2B receptor after 120 mins |
Bioorg Med Chem 24: 1513-9 (2016)
Article DOI: 10.1016/j.bmc.2016.02.021
BindingDB Entry DOI: 10.7270/Q26M38P3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50083351

((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...)Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
I2BM
Curated by ChEMBL
| Assay Description
Antagonist activity at NR1/NR2B receptor assessed as inhibition of Glu/Gly induced Ca2+ influx |
Eur J Med Chem 46: 2295-309 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.013
BindingDB Entry DOI: 10.7270/Q2M32ZMN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50083351

((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...)Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TCG Lifesciences Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 46: 618-30 (2011)
Article DOI: 10.1016/j.ejmech.2010.11.042
BindingDB Entry DOI: 10.7270/Q2WQ052W |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Homo sapiens (Human)) | BDBM50083351

((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...)Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of human GluN2B receptor expressed in xenopus laevis oocytes assessed as reduction in 3 uM glycine-induced channel cur... |
J Med Chem 62: 3-23 (2019)
Article DOI: 10.1021/acs.jmedchem.7b01640
BindingDB Entry DOI: 10.7270/Q2N019RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50083351

((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...)Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
I2BM
Curated by ChEMBL
| Assay Description
Antagonist activity against NR1a/NR2B receptor transfected in human HEK293 cells assessed as inhibition of NMDA-induced Ca2+ influx |
Eur J Med Chem 46: 2295-309 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.013
BindingDB Entry DOI: 10.7270/Q2M32ZMN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50083351

((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...)Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris Descartes
Curated by ChEMBL
| Assay Description
Antagonist activity at wild type NR1/NR2B receptor expressed in Xenopus oocytes assessed as inhibition of agonist-induced current amplitude by two-el... |
Bioorg Med Chem Lett 20: 5552-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.043
BindingDB Entry DOI: 10.7270/Q24M97C9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50083351

((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...)Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
I2BM
Curated by ChEMBL
| Assay Description
Antagonist activity against NR1/NR2B receptor expressed in xenopus oocytes assessed as inhibition of NMDA induced Ca2+ influx |
Eur J Med Chem 46: 2295-309 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.013
BindingDB Entry DOI: 10.7270/Q2M32ZMN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 2C
(Homo sapiens (Human)) | BDBM50083351

((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...)Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of human GluN2C receptor expressed in xenopus laevis oocytes assessed as reduction in 3 uM glycine-induced channel cur... |
J Med Chem 62: 3-23 (2019)
Article DOI: 10.1021/acs.jmedchem.7b01640
BindingDB Entry DOI: 10.7270/Q2N019RG |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase
(Candida glabrata) | BDBM50083351

((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...)Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of human GluN2A receptor expressed in xenopus laevis oocytes assessed as reduction in 3 uM glycine-induced channel cur... |
J Med Chem 62: 3-23 (2019)
Article DOI: 10.1021/acs.jmedchem.7b01640
BindingDB Entry DOI: 10.7270/Q2N019RG |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2D
(Homo sapiens (Human)) | BDBM50083351

((+/-)-[2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-...)Show InChI InChI=1S/C21H27NO2/c1-16(21(24)19-7-9-20(23)10-8-19)22-13-11-18(12-14-22)15-17-5-3-2-4-6-17/h2-10,16,18,21,23-24H,11-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of human GluN2D receptor expressed in xenopus laevis oocytes assessed as reduction in 3 uM glycine-induced channel cur... |
J Med Chem 62: 3-23 (2019)
Article DOI: 10.1021/acs.jmedchem.7b01640
BindingDB Entry DOI: 10.7270/Q2N019RG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data