 Found 12 hits of ic50 for monomerid = 50111445
Found 12 hits of ic50 for monomerid = 50111445 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50111445
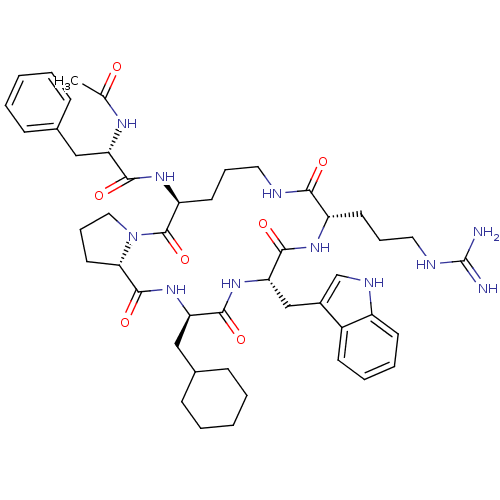
((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I-C5a] from C5a receptor in human PBMC by scintillation counting |
J Med Chem 53: 4938-48 (2010)
Article DOI: 10.1021/jm1003705
BindingDB Entry DOI: 10.7270/Q2QR4X9F |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I-C5a] from C5a receptor in human PBMC by scintillation counting |
J Med Chem 53: 4938-48 (2010)
Article DOI: 10.1021/jm1003705
BindingDB Entry DOI: 10.7270/Q2QR4X9F |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Antagonist activity at C5aR1 in HMDM |
J Med Chem 61: 3253-3276 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00882
BindingDB Entry DOI: 10.7270/Q2MK6GJ9 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR in CD88 transfected RBL cells assessed as inhibition of C5a-induced glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Antagonist activity at C5aR1 in human PMN |
J Med Chem 61: 3253-3276 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00882
BindingDB Entry DOI: 10.7270/Q2MK6GJ9 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Antagonist activity at human C5aR expressed in human PMN cells assessed as inhibition of glucosaminidase release |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to NK2 receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of C5a binding to human C5aR expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
C5a anaphylatoxin chemotactic receptor 1
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity against the C5a receptor |
J Med Chem 45: 1543-58 (2002)
BindingDB Entry DOI: 10.7270/Q2CN74ND |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to MC4 receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to V1a receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50111445

((S)-N-((3R,6S,9S,15S,20aS)-6-((1H-indol-3-yl)methy...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NC(=O)[C@@H]2CCCN2C1=O Show InChI InChI=1S/C47H65N11O7/c1-29(59)53-37(25-30-13-4-2-5-14-30)42(61)55-36-20-11-22-50-41(60)35(19-10-23-51-47(48)49)54-44(63)39(27-32-28-52-34-18-9-8-17-33(32)34)56-43(62)38(26-31-15-6-3-7-16-31)57-45(64)40-21-12-24-58(40)46(36)65/h2,4-5,8-9,13-14,17-18,28,31,35-40,52H,3,6-7,10-12,15-16,19-27H2,1H3,(H,50,60)(H,53,59)(H,54,63)(H,55,61)(H,56,62)(H,57,64)(H4,48,49,51)/t35-,36-,37-,38+,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to ORL1 receptor |
Bioorg Med Chem Lett 16: 5088-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.036
BindingDB Entry DOI: 10.7270/Q2RX9BP8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data