 Found 9 hits of ic50 for monomerid = 50118093
Found 9 hits of ic50 for monomerid = 50118093 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carnitine O-palmitoyltransferase 1, muscle isoform
(Homo sapiens (Human)) | BDBM50118093
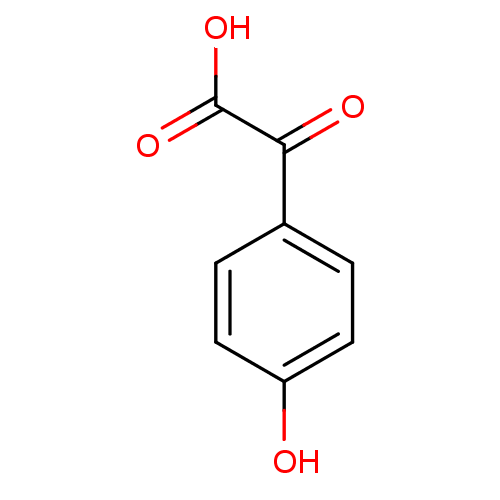
((4-Hydroxy-phenyl)-oxo-acetic acid | CHEMBL129918)Show InChI InChI=1S/C8H6O4/c9-6-3-1-5(2-4-6)7(10)8(11)12/h1-4,9H,(H,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CPT1B in heart mitochondria |
J Med Chem 54: 3109-52 (2011)
Article DOI: 10.1021/jm100809g
BindingDB Entry DOI: 10.7270/Q29K4CDQ |
More data for this
Ligand-Target Pair | |
Carnitine O-palmitoyltransferase 1, muscle isoform
(Homo sapiens (Human)) | BDBM50118093

((4-Hydroxy-phenyl)-oxo-acetic acid | CHEMBL129918)Show InChI InChI=1S/C8H6O4/c9-6-3-1-5(2-4-6)7(10)8(11)12/h1-4,9H,(H,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CPT1B |
J Med Chem 54: 3109-52 (2011)
Article DOI: 10.1021/jm100809g
BindingDB Entry DOI: 10.7270/Q29K4CDQ |
More data for this
Ligand-Target Pair | |
Carnitine O-palmitoyltransferase 2, mitochondrial
(Rattus norvegicus) | BDBM50118093

((4-Hydroxy-phenyl)-oxo-acetic acid | CHEMBL129918)Show InChI InChI=1S/C8H6O4/c9-6-3-1-5(2-4-6)7(10)8(11)12/h1-4,9H,(H,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat CPT2 |
J Med Chem 54: 3109-52 (2011)
Article DOI: 10.1021/jm100809g
BindingDB Entry DOI: 10.7270/Q29K4CDQ |
More data for this
Ligand-Target Pair | |
Carnitine O-palmitoyltransferase 1, liver isoform
(Homo sapiens (Human)) | BDBM50118093

((4-Hydroxy-phenyl)-oxo-acetic acid | CHEMBL129918)Show InChI InChI=1S/C8H6O4/c9-6-3-1-5(2-4-6)7(10)8(11)12/h1-4,9H,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CPT1A |
J Med Chem 54: 3109-52 (2011)
Article DOI: 10.1021/jm100809g
BindingDB Entry DOI: 10.7270/Q29K4CDQ |
More data for this
Ligand-Target Pair | |
Carnitine O-palmitoyltransferase 2, mitochondrial
(Homo sapiens (Human)) | BDBM50118093

((4-Hydroxy-phenyl)-oxo-acetic acid | CHEMBL129918)Show InChI InChI=1S/C8H6O4/c9-6-3-1-5(2-4-6)7(10)8(11)12/h1-4,9H,(H,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CPT2 |
J Med Chem 54: 3109-52 (2011)
Article DOI: 10.1021/jm100809g
BindingDB Entry DOI: 10.7270/Q29K4CDQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50118093

((4-Hydroxy-phenyl)-oxo-acetic acid | CHEMBL129918)Show InChI InChI=1S/C8H6O4/c9-6-3-1-5(2-4-6)7(10)8(11)12/h1-4,9H,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Concentration required for 50% Inhibition of activity against Yersinia Protein-tyrosine phosphatase |
J Med Chem 45: 3946-52 (2002)
BindingDB Entry DOI: 10.7270/Q2F76BW4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118093

((4-Hydroxy-phenyl)-oxo-acetic acid | CHEMBL129918)Show InChI InChI=1S/C8H6O4/c9-6-3-1-5(2-4-6)7(10)8(11)12/h1-4,9H,(H,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Concentration required for 50% Inhibition of activity against Protein Tyrosine Phosphatases 1B |
J Med Chem 45: 3946-52 (2002)
BindingDB Entry DOI: 10.7270/Q2F76BW4 |
More data for this
Ligand-Target Pair | |
Carnitine O-palmitoyltransferase 1, muscle isoform
(Homo sapiens (Human)) | BDBM50118093

((4-Hydroxy-phenyl)-oxo-acetic acid | CHEMBL129918)Show InChI InChI=1S/C8H6O4/c9-6-3-1-5(2-4-6)7(10)8(11)12/h1-4,9H,(H,11,12) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CPT1B in liver mitochondria |
J Med Chem 54: 3109-52 (2011)
Article DOI: 10.1021/jm100809g
BindingDB Entry DOI: 10.7270/Q29K4CDQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F
(Homo sapiens (Human)) | BDBM50118093

((4-Hydroxy-phenyl)-oxo-acetic acid | CHEMBL129918)Show InChI InChI=1S/C8H6O4/c9-6-3-1-5(2-4-6)7(10)8(11)12/h1-4,9H,(H,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Concentration required for 50% Inhibition of activity against Protein-tyrosine phosphatase Lar |
J Med Chem 45: 3946-52 (2002)
BindingDB Entry DOI: 10.7270/Q2F76BW4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data