

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50118810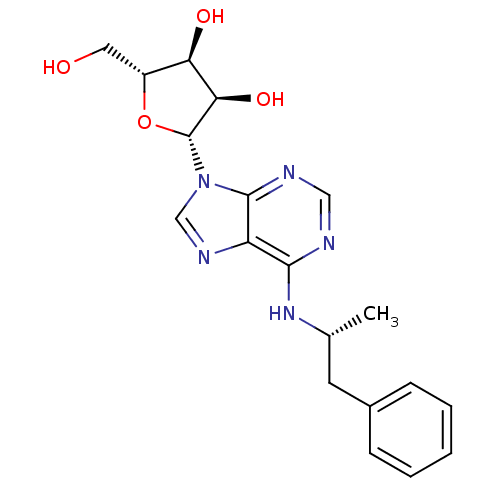 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards the Adenosine A1 receptor in cerebral cortices of Sprague-Dawley male rats using [3H]CHA as radioligand. | J Med Chem 33: 2240-54 (1990) BindingDB Entry DOI: 10.7270/Q2PR7WKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50118810 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of [3H]-CHA binding on rat brain adenosine A1 receptor | J Med Chem 34: 2570-9 (1991) BindingDB Entry DOI: 10.7270/Q2BZ66NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50118810 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition of Adenylate cyclase activity in rat fat cell membrane at adenosine A1 receptor | J Med Chem 31: 1179-83 (1988) BindingDB Entry DOI: 10.7270/Q2QC042G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1/A2a/A2b/A3 (Rattus norvegicus (rat)) | BDBM50118810 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 95.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]CHA from rat brain adenosine receptor | J Med Chem 26: 1601-6 (1983) BindingDB Entry DOI: 10.7270/Q2QR50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1/A2a/A2b/A3 (Rattus norvegicus (rat)) | BDBM50118810 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]CHA from rat brain adenosine receptor | J Med Chem 26: 1601-6 (1983) BindingDB Entry DOI: 10.7270/Q2QR50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50118810 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of [3H]NECA binding on rat brain adenosine A2 receptor | J Med Chem 34: 2570-9 (1991) BindingDB Entry DOI: 10.7270/Q2BZ66NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||